|
|
Email: mfh@chem.ucla.edu |
Boron minerals were discovered in what we now know as Southern California about 1869, and there are many, many localities in the state where boron is available, but three are worth mentioning. The Harmony Borax Works in Death Valley was one of the original sources of borate. Searles Lake is a dry lake that hasn't quite dried yet. It has brine rich in boron that's processed for boric acid. And there is the world's largest open pit mine, owned by U.S. Borax. About half of the mined borax used each year in the world comes from this site. That's a lot of boron: about 500,000 metric tons per year.Perhaps you're familiar with 20 Mule Team Borax, the trademark of U.S. Borax. The 20-mule teams at Harmony Borate Works in Death Valley would take a wagon of borate ore 165 miles to Mojave, Calif., to the railhead, at which point it was transferred to rail cars and shipped out for industrial purposes. The story is that after many, many years of operation, not a single mule was lost. It was all very humane.In the 1950s U.S. Borax, by way of advertising, produced a TV series called "Death Valley Days." It was a Western-type drama in which the good guy always won. The good guy was Ronald Reagan. One could say the program's popularity contributed somewhat to his popularity as a human being and as an actor, and certainly helped him in his political career. Now, let's talk a little bit about boron itself. We know boron likes to combine with oxygen and form borates such as borax, boric acid and boric anhydride. We know carbon, next to boron on the Periodic Table, combines readily with oxygen to form carbon monoxide and carbon dioxide. We also know carbon combines with hydrogen to form hydrocarbons, such as the gasoline that goes into our gasoline tanks. 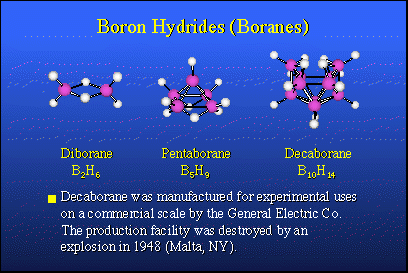
In nature, there are no equivalent boron-hydrogen compounds and there is no petroleum analog for boron. Therefore, about 1910, Alfred Stock in Karlsruhe, Germany, took it upon himself to explore the possibility of preparing boron hydrogen compounds, so-called boron hydrides, or boranes, which would be analogous to hydrocarbons. He succeeded in making small quantities of very expensive, very rare compounds. Their chemistry was not well understood until about 1950. The nice thing about these materials is that when they burn, they release large quantities of heat and they would make excellent fuels if one had sufficient quantities of them.In 1946, following the conclusion of World War II, Project Hermes was organized by the U.S. Army. The purpose was to synthesize boron hydrides in commercial quantities and perhaps use them as fuels in rockets. The heat of combustion of boron hydrides is typically about 30,000 BTUs per pound, while a hydrocarbon fuel, such as JP4 or kerosene, was about 18,000 BTUs. This was a tremendous step up in potential energy content, and it was attractive to people designing rocket engines and weapon systems.Accordingly, General Electric Company undertook a research and development program in Malta, N.Y., which actually made commercial quantities, several hundred pounds of diborane, pentaborane and decaborane. These more stable boron hydrides are the contributions of Alfred Stock. All went well until about 1948, when the plant was cleaned and washed out with carbon tetrachloride, not realizing that carbon tetrachloride and decaborane form an explosive mixture equivalent to nitroglycerin. The plant was blown away. So, live and learn. Anton Burg at USC had warned them that this might happen. They did it nonetheless.That didn't change things and the rocket program went ahead. In the meantime, a new bomber was designed, the B-70 Valkyrie, an intercontinental bomber capable of reaching the Soviet Union and returning to the United States without midair refueling. To do this, it required boranes as fuels to operate the turbojet engines. Millions and millions of dollars were put into a fuel program as well as the bomber itself.After about 10 years of research, it was found that you just could not make a turbine engine strong enough to stand up to the boron oxide and boron carbide products of combustion formed from the fuels, so this project was dropped and the B-70 reverted to hydrocarbon fuels. The rocket fuel program floundered. The problem was that the solid boron oxides present in the combustion products of the rocket motor, interfered with the expected thermodynamics. So boranes were not super fuels for rockets and that program was dropped. An unexpected finding, however, was that solid propellants based upon boron hydrides have a very high burning rate and this makes them attractive for anti-ballistic missile defense rockets and other things that require rapid response. That application still exists today, although nothing is really being done about it. |