Chlorophyll
The synthesis of chlorophyll (Chl) is a major metabolic pathway in photosynthetic organisms. The pathway is rather well studied through genetic analysis of mutants and by in vitro reconstitution of individual reactions. In brief, the pathway involves condensation of 2 molecules of δ-aminolevulinic acid (δ-ALA) to yield a pyrrole intermediate, porphobilinogen, which is then converted to a tetrapyrrole that is in turn the substrate for ring closure and formation of a cyclic tetrapyrrole, uroporphyrinogen III. All natural tetrapyrroles are derived from uroporphyrinogen III. Side chain decarboxylations and ring oxidations lead to protoporphyrin IX (ProtoIX), which can be converted to heme via the action of ferrochelatase, or to Mg-ProtoIX, the substrate for chlorophyll and bacteriochlorophyll synthesis.
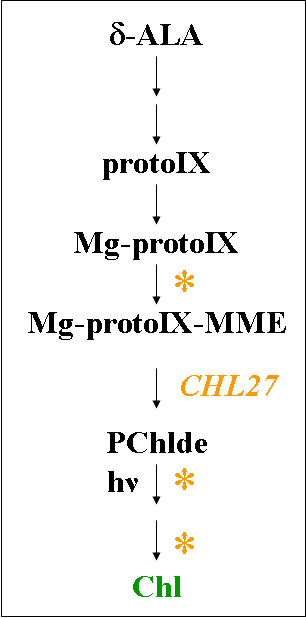 |
| Figure 1: The Mg branch of the tetra-pyrrole pathway. |
The key steps from Mg-ProtoIX to Chl a include oxidative cyclization to form the isocyclic ring, reduction to form chlorophyllide, side chain modification and esterification. At the start of the sequence of reactions, the carboxylate of the propionate side chain that provides the skeleton for the exocyclic ring is protected by methylation in an S-adenosyl methionine-dependent reaction to yield Mg-ProtoIX monomethyl ester (MME). There are two alternative means by which the isocyclic ring E is generated. In anaerobic cells, the bchE gene product, a cobalamin-dependent enzyme, catalyzes the cyclization reaction via radical chemistry. The keto oxygen atom of the isocyclic ring is derived from water. Under aerobic conditions, this oxygen atom is derived instead from molecular oxygen through the action of an Fe-dependent oxygenase. Although an experimentally based reaction pathway has been proposed, the mechanistic details have not yet been described because the components of the enzyme are not known. The product of the cyclase, divinyl protochlorophyllide (Pchlde), is reduced to monovinyl-Pchlde. The position of the divinyl reductase reaction in the pathway remains open for discussion. It may occur prior to completion of the cyclase step, immediately after, or even after formation of chlorophyllide (Chlde). The conversion of Pchlde to Chlde is another reaction that can occur by one of two means: in a light-dependent reaction or a light-independent one. The enzyme NADPH-Pchlde oxidoreductase (POR) has a photochemical requirement for light. It is the only enzyme found in angiosperms, and hence chlorophyll synthesis in these organisms is completely light dependent. An entirely different 3-subunit enzyme functions in the light-independent pathway in other photosynthetic eukaryotes and in various photosynthetic bacteria. In the eukaryotes these subunits appear to be encoded in the plastid genome. After formation of Chlde, the remaining free carboxylate on Ring D of Chlde a is esterified to yield Chl a.
THE AEROBIC CYCLASE
Of all the enzymes in chlorophyll biosynthesis, the aerobic oxidative cyclase is the most poorly understood at the molecular level. One may attribute this to the fact that the prototypical pathway was derived from the very elegant and efficient dissection of the bch genes in the photosynthetic gene cluster of the model organism Rhodobacter capsulatus, which does not require an aerobic cyclase because it synthesizes Bchl only under anaerobic conditions.
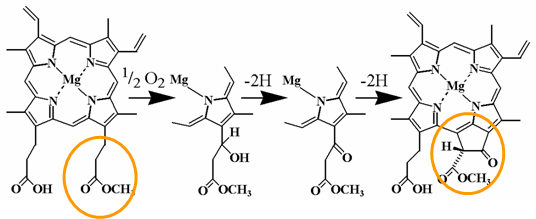 |
| Figure 2: Proposed pathway for the overall aerobic cyclase reaction. |
The first step is the stereospecific hydroxylation of the methyl esterified Ring C propionate, followed by oxidation of the alcohol to the corresponding ketone. The now activated methylene group then reacts with the γ meso carbon of the porphyrin nucleus in an oxidative reaction involving removal of 2H to yield Ring E. The hydroxylated compound was suggested to be a genuine intermediate based on its pattern of accumulation during the course of the reaction – an initial increase followed by a decrease as product was formed – and because it could serve as a substrate for the enzyme. In the same studies from the Castelfranco group, the keto compound was likewise established as an intermediate on the basis of its ability to serve as a substrate for the enzyme. An Fe requirement in the aerobic cyclase reaction was proposed some time ago because iron-deficient plants accumulate pathway intermediates such as MgProtoIX and MgProtoIXMME, and this was confirmed subsequently by inhibition of enzymatic activity in vitro by iron chelators.
CHL27
In collaboration with Poul Erik Jensen’s group in Copenhagen, we established that a new di-iron enzyme (whose occurrence is restricted to organisms that have the capacity to make Chl (or Bchl) under aerobic conditions) is required for conversion of MgProtoIXMME to DV-Pchlde (Moseley et al., 2000; Tottey et al., 2003). The corresponding gene was named CHL27. Based on the reactions catalyzed by the aerobic oxidative cyclase and in vitro reconstitution of the reaction from wild-type and mutant extracts by von Wettstein and co-workers, it is apparent that the cyclase is a multi-subunit membrane protein complex. A key objective of this project is the resolution of additional subunits. In Chlamydomonas, the di-iron protein occurs in two forms: Chl27A expressed in –Cu cells and Chl27B expressed in +Cu cells. They are functionally analogous but not identical. A second objective in this project is to a) distinguish whether the two proteins are differently localized, and b) differentiate between the phenotypes of chl27A and chl27B strains.

References
1. Quinn, J.M., Barraco, P., Eriksson, M., Merchant, S. (2000) Coordinate copper- and oxygen-responsive Cyc6 and Cpx1 expression in Chlamydomonas is mediated by the same element. J. Biol. Chem 275:6080-6089.
2. Tottey S., Block, M.A., Allen, M., Westergren, T., Albrieux, C., Scheller, H.V., Merchant, S., Jensen, P.E. (2003) Arabidopsis CHL27, located in both envelope and thylakoid membranes, is required for the synthesis of protochlorophyllide. Proc. Natl. Acad. Sci. USA 100:16119-16124