Iron
Iron-deficiency limits photosynthetic productivity on land and in the oceans: Although iron is an abundant element on earth, iron nutrition is one of the widest ranging limitations to life. This is because of poor bioavailability, which is exacerbated in an alkaline environment by the low solubility of Fe3+ oxides. Plants have devised various and multiple strategies for acquiring iron, including energy dependent acidification of the soil, iron reduction and high affinity Fe2+ transport, and phytosiderophore secretion and uptake of Fe-siderophore complexes. Nevertheless, iron-deficiency chlorosis remains an agricultural problem. Iron-deficiency also limits ocean productivity. Ocean fertilization experiments completed in the last decade have shown that iron limitation of phytoplankton growth occurs not only in the high nutrient low chlorophyll regions of the equatorial Pacific and northeast Pacific sub arctic areas but also in the low nutrient South Pacific where nitrogen is assumed to be limiting. This means that photosynthesis in the oceans and also on land occurs in an iron-limited environment; yet our understanding of photosynthetic mechanisms is based on an apparatus assembled in the laboratory in artificial nutrient rich medium.
 |
|
| Figure 1. Iron deficiency is definted by marker gene expression and uncoupled photosynthetic antenna but normal growth rate. Iron limitation is defined by chlorosis and inhibited cell division. |
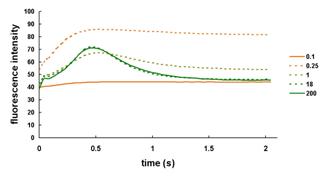 |
|
|---|---|
| Figure 2. Fluorescence and decay kinetics show a progressive loss of photosystem function as cells get more Fe-deficient. PSI is affected before PSII. |
Chlamydomonas responds to iron nutrition status by graded changes: We can assess iron nutritional state in Chlamydomonas based 1) on the pattern of expression of genes encoding iron assimilation components and 2) on the operation of the photosynthetic apparatus (La Fontaine et. al, 2002; Moseley et. al, 2002). On this basis we define three nutritional states for the proposed analytical work: iron replete, corresponding to a concentration of 18 – 200 μM Fe-EDTA in the medium, iron deficient, corresponding to a concentration of 1 μM for mixotrophic growth in acetate containing medium, and iron-limited, corresponding to 0.1 to 0.5 μM. Our previous studies indicate that adaptation to Fe-deficiency involves sequential and progressive modifications to the photosynthetic apparatus based on the severity of the deficiency. The physiological function of the response is to prevent photooxidative damage resulting from inhibition of electron transfer.
We are now dissecting the Fe-deficiency response in the context of chloroplast-based photosynthesis by using genomic tools in combination with classical genetic methodology in Chlamydomonas. The long term goal is to understand the sequential and progressive modifications of the photosynthetic apparatus in response to iron nutritional state.

References
1. LaFontaine, S., Quinn, J.M., Nakamoto, S.S., Page, M.D., Göhre, V., Moseley, J.L., Kropat, J., Merchant, S. (2002) Copper-Dependent Iron Assimilation Pathway in the Model Photosynthetic Eukaryote Chlamydomonas reinhardtii. Eukaryotic Cell 1(5):736-757.
2. Moseley, J., Allinger, T., Herzog, P., Wehinger, E., Merchant, S., Hippler, M. (2002) Adaptation to Fe-deficiency requires re-modelling of the photosynthetic apparatus. EMBO J. 21: 6709-6720.