b. 1939) A.B. 1960, Oberlin College; A.M. 1962, Ph.D. 1965, Harvard University; N.I.H. Postdoctoral Fellow 1965-68, Harvard Medical School; Visiting Scientist 1975-76, Oxford University; Visiting Scientist 1984, Pasteur Institute; Alfred P. Sloan Fellow, 1972-74; Josiah Macy Jr. Foundation Faculty Scholar, 1975-76; McCoy Research Award,1991. B.R. Baker Lecturer, University of California, Santa Barbara (1996).
Our research group works at the interface of bioorganic chemistry and molecular biology to accomplish the following goals:
a) Synthesis of Site-Specific Nucleases and Proteases
b) Design of Gene-Specific Inhibitors of Transcription;
c) Development of Sequence-dictated DNA Cleavage Methodologies to Analyze Genome Structure.
Chemical nucleases are artificial catalysts which cleave the phosphodiester backbone of DNA and RNA by oxidative attack on the ribose moiety. Our laboratory discovered the first reagent of this type, the 2:1 1,10-phenanthroline-cuprous which acts with hydrogen peroxide as a coreactant. This simple coordination complex and its derivatives cleave double-stranded DNA and single stranded regions of RNA.
The specificity of the cleavage reaction can be modified by linking 1,10-phenanthroline-copper to ligands with specificity for different aspects of DNA structure. Our research is currently focused on converting DNA binding proteins into site specific scission reagents. Site-directed mutagenesis is used to convert amino acids residues adjacent to the DNA to cysteines which are then alkylated by 5-iodoacetamido-1,10-phenanthroline. These chimeric proteins cleave DNA with a specificity dictated by the binding specificity of the carrier protein. These experiments can be used to test structural models of the interaction of the DNA binding protein with its recognition sequence, to discover the location of binding sites for different regulatory proteins within genomes and to generate a new family of highly specific "restriction enzymes".
The reactive oxidative species formed by 1,10-phenanthroline copper also cleaves pep tide bonds. As a result, the topography of proteins can be mapped when 1,10-phenanthroline is tethered to a specific sequence position. This new method of chemical crosslinking is being applied to membrane proteins such as the lac permease. The mechanism of the proteolytic cleavage is under investigation.
b) Design of Gene-Specific Inhibitors of Transcription
The synthesis of RNA from DNA requires that RNA polymerases tease apart the two interwoven strands of the DNA duplex to allow the faithful copying of the nucleotide sequence of the template. These transient single-stranded structures of DNA formed at the active site of RNA polymerases during catalysis are only generated in actively transcribing genes and therefore provide unique targets for inhibitors of gene expression.
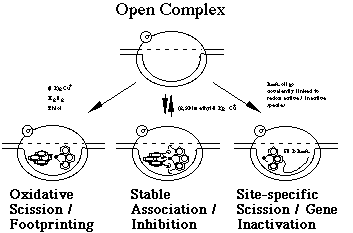
Our laboratory has developed two types inhibitors which bind to these enzyme-bound single-stranded structures at the initiation site of RNA synthesis. One class of inhibitors is the tetrahedral redox stable cuprous complexes of neocuproine and its derivatives. The second are oligoribonucleotides whose sequences are complementary to the template strand of the DNA transcribed. As indicated in the accompanying figure, the open complex is also the target of the chemical nuclease activity of the 2:1 1,10-phenanthroline-cuprous complex discussed above. The techniques which we are use in our study of the mechanism of action of these inhibitors are chemical synthesis, spectroscopy, site-directed mutagenesis and enzyme assays. In addition to contributing to our understanding of the mechanism of action of RNA polymerase, possible practical implications of this research would be the development of gene-specific inhibitors of transcription which could serve as antiviral and antibacterial agents.

c) Site Directed Protein Cleavage
The 1,10-phenanthroline-copper complex also catalyzes the oxidative scission of peptides by hydrogen peroxide. When linked to a protein either covalently or by a reversibly binding ligand, scission of neighboring peptide bonds is achieved providing a novel method of chemical crosslinking. This chemistry is being applied to study the structure of the lac permease and of bacterial invertasomes which are composed of the Fis and Hin proteins .
1. Sigman, D. S., Graham, D. R., D'Aurora, V., and Stern, A. M. (1979) "Oxygen-dependent Cleavage of DNA by the 1,10-Phenanthroline-Cuprous Complex. Inhibition of Escherichia coli DNA Polymerase I". J. Biol. Chem. 254, 12269-12272.
2 Pope, L. E. and Sigman, D. S. (1984) "Secondary Structure Specificity of the Nuclease Activity of the 1,10-Phenanthroline-Copper Complex". Proc. Natl. Acad. Sci. USA 81, 3-7.
3. Spassky, A. and Sigman, D. S. (1985) "Nuclease Activity of 1,10-Phe nanthroline-Copper Ion. Conformational Analysis and Footprinting of the lac Operon". Biochemistry 24, 8050-8056.
4. Chen, C.-h. B. and Sigman, D. S. (1986) "Nuclease Activity of 1,10-Phenanthroline-Copper: Sequence-Specific Targeting". Proc. N atl. Acad. Sci. USA 83, 7147-7151.
5. Goyne, T. E. and Sigman, D. S. (1987) "Nuclease Activity of 1,10-Phenanthroline-Copper Ion. Chemistry of Deoxyribose Oxidation". J. Am. Chem. Soc. 109, 2846-2848.
6. Chen, C.-h. B. and Sigman, D. S. (1987) "Chemical Conversion of a DNA-Binding Protein into a Site-Specific Nuclease". Science 237, 1197-1201.
7. Sigman, D.S., Bruice, T.W., Mazumder, A., and Sutto n, C.L. (1993) "Targeted Chemical Nucleases". Acc. Chem. Res., 26, 98-104.
8. Sigman, D.S., Chen, C.-h.B., Gorin, M.B. (1993) "Sequence-Specific Scission of DNA by RNAs Linked to a Chemical Nuclease" Nature 363 474-475.
10. Mazumder, A., Per rin, D.M., Watson, K.H. and Sigman, D.S. (1993) "A Transcription Inhibitor Specific for Unwound DNA in RNA Polymerase-Promoter Open Complexes". Proc. Natl. Acad. Sci USA., 90, 8140-8144.
11. Perrin. D.M., Mazumder, A., Sadeghi, F. and Sigman, D.S.(1994) "Hybridization of a Complementary Ribooligonucleotide to the Transcription Start Site of the lacUV-5-Escherichia coli RNA Polymerase Open Complex. Potential for Gene- Specific Inactivation Reagents". Biochemistry 33, 3848-3854.
12. Pan, C. Q., Landgraf, R., and Sigman, D. S. (1994) " DNA Binding Proteins as Site-Specific Nucleases". Molecular Microbiology 12, 335-342.
13. Landgraf, R., Chen, C.-h. B., and Sigman, D. S. (1995)"Double-Stranded Cleavage of DNA at any Preselected Sequence". Nucleic Acids Research 23, 3524-3530.
14. Pan, C.Q, Finkel, S.E., Cramton, S.E., Feng, J-A., Sigman, D.S,. Johnson, R.C. (1996)" Variable Structures of Fis-DNA Complexes Determined by Flanking DNA-Protein Contacts". J. Mol. Biol. 264, 675-695.
15.Meijler, M., Zelenko, O, Sigman, D.S. (1997) "Chemical Mechanism of DNA Scission by 1,10-Phenanthroline-Copper. Carbonyl Oxygen of 5-Methylene Furanone Is Derived From Water." J. Amer. Chem. Soc. 119 1135-1136.
16. Perrin, D.M., Chen, C-h.B., Xu, Yue, Pearson, L., and Sigman, David S.(1997) " Gene Specific Transcription Inhibitors. Oligonucleotides Complementary to the Template Strand of Transcription Start Sites."J. Amer. Chem. Soc. 119 5746-5747.
[ Department * Faculty* Current Organic Research* Sigman Biography* Organic Research Interests ]
Last Revision: 08/23/95 // burns@chem.ucla.edu