 |
|
Reaction mechanisms, novel materials, and chemical communication comprise our research interests. Our recent work on reaction mechanisms has focused on divalent carbon and univalent nitrogen chemistry and elucidation of the mechanism of arylmethylene rearrangements. These mechanisms include ring expansion of arylmethylenes to cycloheptatetraenes. Argon matrix spectroscopy plays an important role in our mechanistic investigations, and photochemistry provides many challenges. We also use matrix techniques to prepare and characterize unusual molecules of theoretical interest. The parent monomer of graphite, C6 and dodecadehydrodiamantane represent two examples of current problems. Our interest in novel materials derives from our interest in novel molecules and emphasizes physical properties. We seek to prepare organoferromagnets and materials with unusual strength and hardness. One major goal is the rational synthesis of spherical C80, which we expect to have most unusual properties. Carbons of all kinds fascinate us. Particulate carbon emissions from gasoline engines pose an increasing health hazard; these particles react with almost anything including air. These reactions produce diazocompounds, carbonyl oxides, perepoxides, and other dangerous materials. Matrix techniques also serve in determining these chemical processes. In the biological area, we study chemical communication: insect-insect communication, insect-plant, plant-plant, and cell-cell communication. At this time , we are developing materials that resist macrophage assault when implanted in human bodies, and we are designing solid materials that possess immune recognition sites. Our newest interest, chemical robotics, involves building robots that do synthesis.
We developed special techniques for the synthesis (photochemical and thermal) characterization of reactive molecules in an argon matrix at 4-10K. Recent successes are shown in
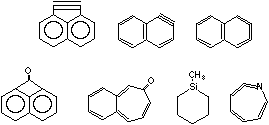
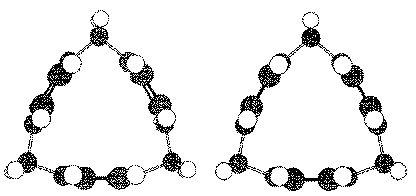
Synthesis of molecular forms of pure carbon pose particular challenges. Cyclic-C6 should have an aromatic, six-electron in-plane system as well as the traditional, benzene p-system. The soccer ball (spherical C-60) will be the first molecular form of carbon stable at room temperature .
Our success in characterization of cycloheptatetraene led to detailed investigations of the mechanism of arylmethylene rearrangements and these inturn led us to the investigation of the mechanism by which carbon atoms are delivered from high defect carbon surfaces to organic molecules. Other mechanism problems of current interest involve isoacenotropones, trans-cyclo-hexenes, and dehydroaromatics. Isotopic labeling [2]H, [13]C, [18C] plays a major role in our studies of rearrangement mechanisms.
We are currently active in three areas of bio-organic chemistry. DNA-small molecule binding, proximity labeling, and chemical communication. Our long range goal in the DNA chemistry is a molecular -level understanding of the binding of small molecules which will permit the design and synthesis of a non-natural anti-tumor antibiotic. Our major success in this area has been the synthesis and evaluation of a bis-psoralen

which is active against squamous cell carcinoma. Proximity labeling is a new method developed in our group for establishing proximity between macromolecules of biological importance (two proteins on a membrane for example) and for probing proximity between different regions of the same macromolecule. The system consists of an anchor (covalent bond to the macromolecule), a tether which permits a useful search radius, and a photo-activated group which will react only with itself. Our chemical communication research has focused on insect pheromones. Recently we used chiral mimics of achiral pheromones to probe neurophysiological aspects of perception, and we have isolated, characterized, and synthesized the pheromones of Heliothis zea and Heliothis Virescens, the two most economically damaging crop insects worldwide. Our current work is focused on human pheromones.
As our microtechniques for identification and characterization become better we are turning to the isolation and characterization of lipids which are important as hormones and in the immune system. We can analyze lipids at the picogram (10[-12]level, and we have characterized insect pheromones when only a few nanograms (10[-9]g) were available.
In polymer chemistry, we design and synthesize
novel materials and demonstrate radically new methods. We are
interested in the linear polyacenes as possible superconductors
and ferromagnetic organics. The molecular soccer ball (spherical
C-60_ is a special molecular, conducting system. The iso-acenotropones,
which we discovered, find applications as conducting systems and
in the shaping of polymers. These shape-selective cavities are
used in chromatography and offer special opportunities in synthesis
of shape-selective organic catalysts.
(1) S. Kivelson and O. L. Chapman, "Polyacene and a New Class of Quasi- One Dimensional Conductors", Physical Review B. 28, 7236 (1983).
(2) O. L. Chapman and T. C. Hess, "Cyclopentadienone
O-Oxide: Spectroscopic Observation and Photochemistry of a Carbonyl
Oxide", J. Am. Chem. Soc.
106, 1842 (1984).
(3) O. L. Chapman, R. J. McMahon and
P. R. West, "Rearrangements of Tosylmethylenes via Cycloheptatetraenes:
Formation of Benzocyclobutene and
Styrene:, J. Am. Soc. 106, 7973 (1984).
(4) O. L. Chapman and U.-P. Eric Tsou,
"Mechanism of the Thermal Isomerization of Benzocyclobutene
to Styrene", J. Am. Chem. Soc. 106, 7974
(1984).
(5) R. J. McMahon, O. L. Chapman, R.
A. Hayes, T.C. Hess, and H.-P. Krimmer, "Mechanistic Studies
on the Wolff Rearrangement: The Chemistry and
Spectroscopy of Some [[alpha]]-Ketocarbenes", J. Am. Chem.
Soc. 107, 7597 (1985).
(6) R. J. McMahon and O. L. Chapman,
"Triplet Ground-State Cycloheptatrienylidene", J. Am.
Chem. Soc. 108, 1713 (1986). < p> (7) O. L. Chapman and
A.
A. Russell, "Imaging Training Via Interactive Video",
Advanced Imaging 5, 28 (1990).
(8) O. L. Chapman and A. A. Russell, "Structure, Chirality, and FT-NMR in Sophomore Organic Chemistry", J. Chem. Ed., 69, 779-782 (1992).
(9) Ke-Jian Fu, William L. Karney, Orville
L. Chapman, Shiou-mei Huang, Richard B. Kaner, Francois Diederich,
Karoly Holczer, Robert L. Whetten,
"Giant vibrational resonances in A6C60 Compounds." Physical
Review Lett ers, 46(3), 1937-1940 (1992).
|
Email: olc@chem.ucla.edu Phone: 310-825-4883 Fax: 310-267-2288 |
University of California, Los Angeles
Department of Chemistry and Biochemistry 607 Charles E. Young Drive East Los Angeles, CA 90095-1569 |

Updated on 10/1/99 by Alice Ramirez: alice@chem.ucla.edu