 |
 |
Miguel A. Garcia-GaribayB.Sc., 1982, Universidad Michoacana, Mexico;
Ph.D., 1988,
|
 |
 |
Miguel A. Garcia-GaribayB.Sc., 1982, Universidad Michoacana, Mexico;
Ph.D., 1988,
|
Most of us have experienced the fascination of growing crystals. However, it is not commonly recognized that organic crystals may offer some of the most efficient methodologies for controlling chemical reactivity and a unique opportunity to explore the most intimate structural details of selected organic reaction mechanisms. Our group is dedicated to uncover the factors that control the chemical reactivity and physicochemical properties of organic crystalline compounds.
|
The study of chemical reactivity in organic crystals offers a wealth of structural information. The structures of a reactant and its environment before reaction can be obtained from X-ray diffraction data. Crystal Chemistry. We design suitable reaction models to test structural and mechanistic aspects of organic reactivity, prepare crystalline compounds to carry out our reactions, and carry out spectroscopic analyses and computer modeling to round up our investigations. Our studies include both thermal and photochemical reactions with emphasis in reactions that involve highly reactive and preferably irreversibly formed intermediates (e.g. carbenes, biradicals, etc.). We select reactions that may occur through common intermediates originating from different precursors to address the role of structural and energetic differences. Carbenes, for instance, may be generated from diazo compounds, from diazirines, from aromatic epoxides, from small ring ketones and from organometallic compounds. By preparing sets of reactants with substituents that are too remote to cause any change in reactivity in solution, but that are able to modify the crystal structure, we address the role of crystal forces in determining the reactivity observed.
|
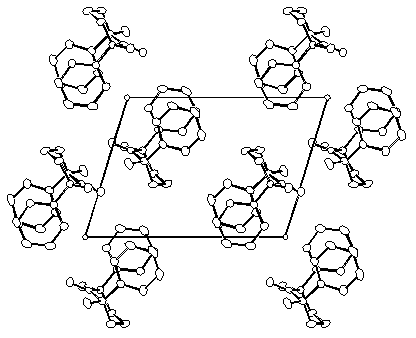 |
Stereoselectivity, Stereospecificity and Chemical Dynamics in Organic Crystals. We address challenging aspects of stereochemical control such as the generation of optically active products from achiral (or even racemic!) starting materials without using optically active reagents! Possible mechanisms for the generation and amplification of optical activity using chiral crystals are relevant to the prebiotic origin of optical activity on earth which we explore with crystalline model systems. We follow the course of our solid state reactions by X-ray crystallographic analysis and rationalize aspects such as stereoselectivity and stereospecificity using computer modeling to aid our analysis. Time-resolved spectroscopic techniques are used in our group for measuring reaction rates that allow us to carry out deeper structure-reactivity correlations based on absolute reaction dynamics. Recent structure-reactivity correlations studies in solids have led us to uncover several interesting aspects of quantum mechanical tunneling reactions.
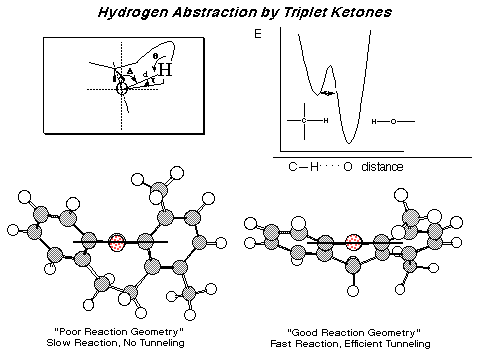
|
Organic Crystals in Material Sciences. Non-linear optics, organic ferromagnets, organic conductors, and piezoelectric materials are some of the newer technological applications of organic crystals. Our group brings a physical organic point of view to aspects relevant to material science. We focus on problems related to the formation, chemical stability, phase changes, and spectroscopic properties of crystals. Two of the main challenges in the field relate to the rational design of crystals with desirable properties and to the manipulation and design of multicomponent solid systems. We investigate the connection between molecular structure and crystal structure by studying the effect of substituents on the crystal structure of a parent compound. Mixed crystals or solid solutions are useful both in addressing mechanistic questions and in learning the properties of multicomponent materials. This research contributes to the challenging area of crystal engineering and is an important aspect of our work. |
Garcia-Garibay Group
University of California, Los Angeles
|
Ng, D; Yang, Z; Garcia-Garibay, MA.
"Engineering reactions in crystals: gem-dialkoxy substitution
enables the photodecarbonylation of Levitus, M; Schmieder, K; Ricks, H; Shimizu,
KD; Bunz, UHF; Garcia-Garibay, MA. "Steps to demarcate the
effects of chromophore aggregation and planarization in poly(phenyleneethynylene)s.
1. Rotationally interrupted conjugation in the excited states
of Dominguez, Z; Dang, H; Strouse, MJ; Garcia-Garibay,
MA. "Molecular "compasses" and "gyroscopes."
- III. Dynamics of a phenylene rotor Campos, LM; Dang, H; Ng, D; Yang, Z; Martinez,
HL; Garcia-Garibay, MA. "Engineering reactions in crystalline
solids: Predicting photochemical decarbonylation from calculated
thermochemical parameters" J. Amer. Chem. Soc., 2002,
V67, 3749-3754. Schmieder, K; Levitus, M; Dang, H; Garcia-Garibay, MA. "Photophysical properties of coplanar and twisted 1,4-bis(9 ethynylanthracenyl)benzene. Rotational equilibration in the excited states of diaryalkynes" J. Phys. Chem. A, 2002, V106,1551-1556. Dang, H; Levitus, M; Garcia-Garibay, MA.
"One step Pd(0)-catalyzed synthesis, X-ray analysis, and
photophysical properties of Ng, D; Yang, Z; Garcia-Garibay, MA. "Engineering
reactions in crystals: suppression of photodecarbonylation by
intramolecular Johnson, BA; Hu, YF; Houk, KN; Garcia-Garibay,
MA. "Vibrationally assisted tunneling in a hydrogen atom
transfer reaction" Garcia-Garibay, MA; Ramamurthy, V; Scheffer,
JR. "Molecular assembly and reactivity of organic crystals
and related structures" Garcia-Garibay, MA; Houk, KN; Keating, AE; Cheer, CJ; Leibovitch, M; Scheffer, JR; Wu, LC. "Computational prediction of the enantioselectivity of a solid-state photoreaction" Org. Letts., 1999, V1, 1279-1281. |
| Email: mgg@chem.ucla.edu Phone: 310-825-3159 Fax: (310) 825-0767 |
University of California,
Los Angeles Department of Chemistry and Biochemistry 607 Charles E. Young Drive East Los Angeles, CA 90095-1569 |
updated 2/13/03 by Alice Ramirez