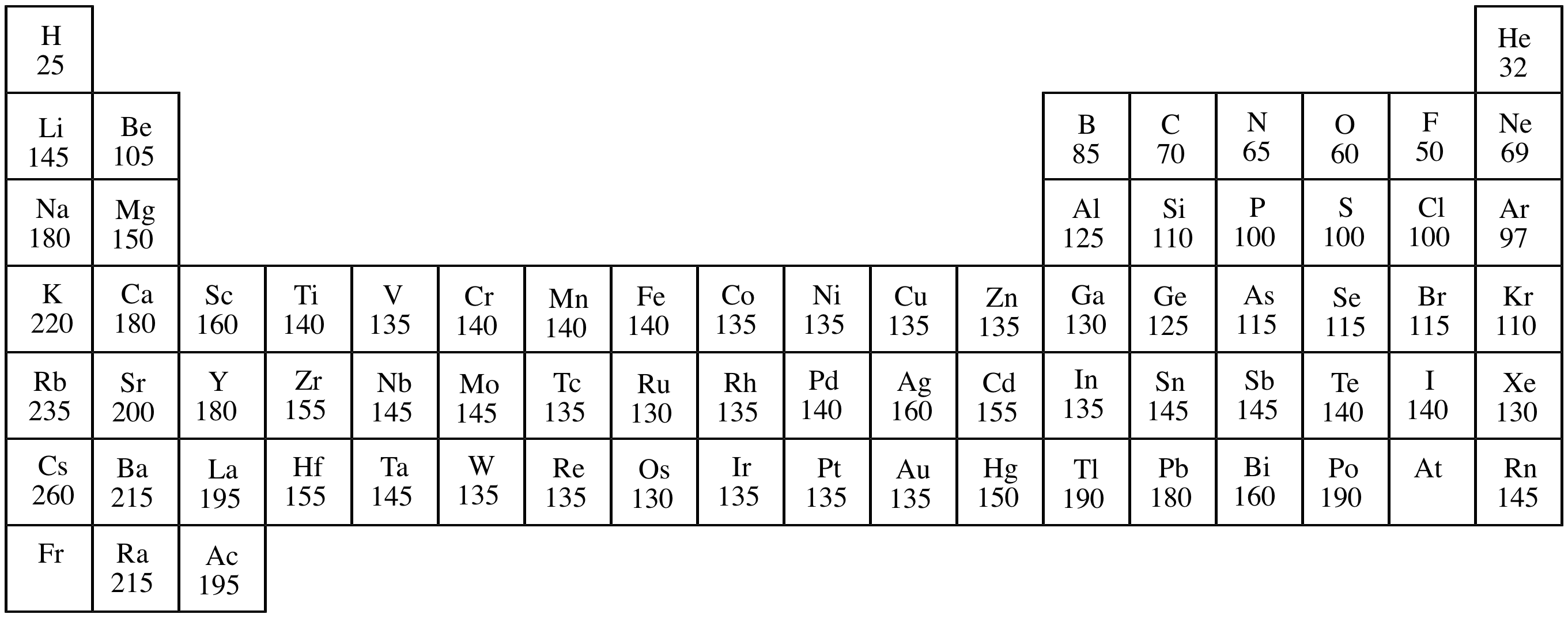
Atomic radius:
The radius of an atom. This distance between an atom's
nucleus and outer electron shell. (This is not a fixed entity, so
there are numerous definitions of this term, depending upon the
measurement used.) Atomic radius differs with the bonding state of
an atom (for example an nonbonded atom of an element versus the
same element within a
covalent
bond). Atomic radius influences many physical and chemical
properties, such as
polarizability,
nucleophilicity,
London
forces, etc.