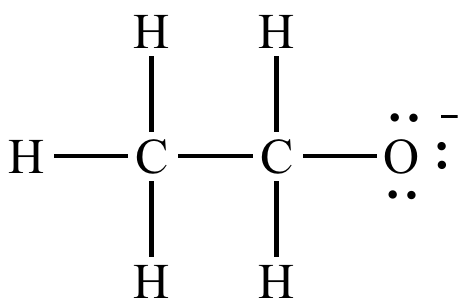
The oxygen lone pairs of ethoxide ion are not delocalized.
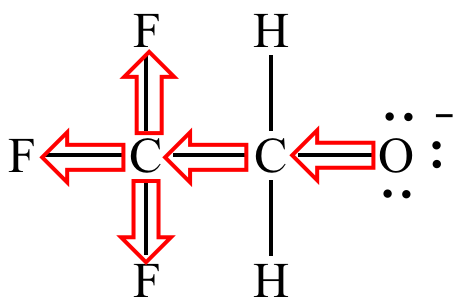
The oxygen lone pairs of trifluoroethoxide ion are delocalized via the trifluoromethyl group's electron-withdrawing inductive effect (symbolized by arrows).


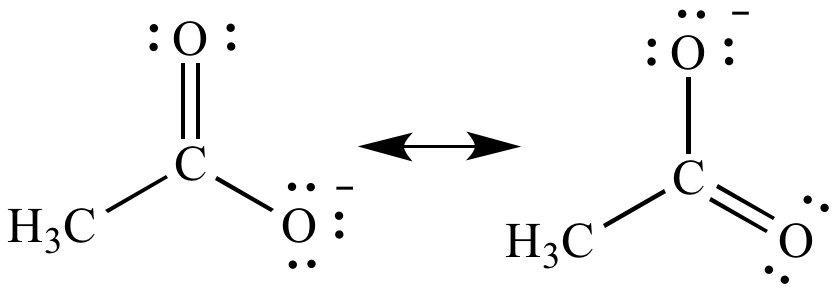 |
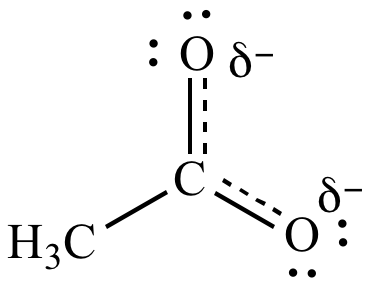 |
|
| Resonance
contributors |
|
Resonance
hybrid |
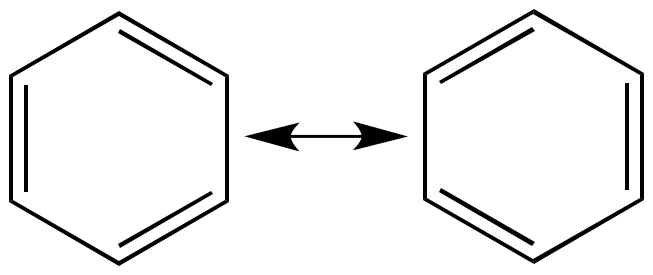 |
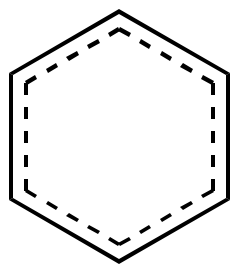 |
|
| Resonance
contributors |
|
Resonance hybrid |