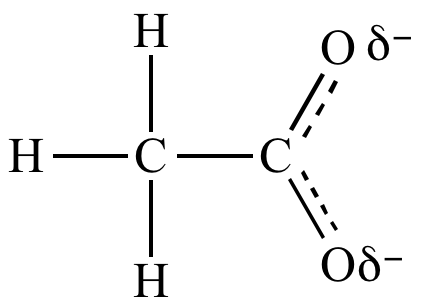
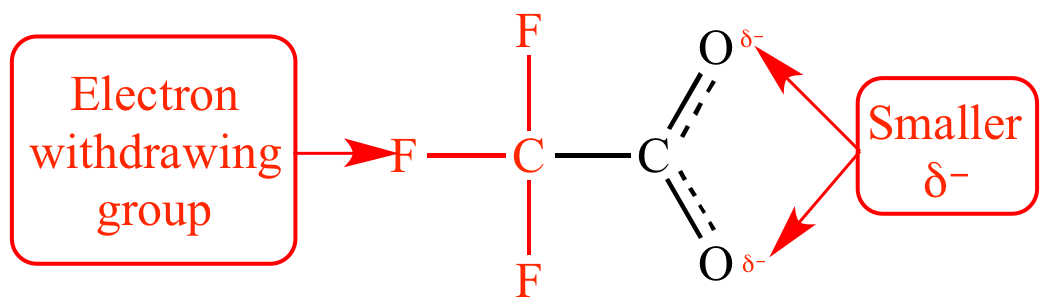
Trifluoroacetate
ion is a weaker base
than acetate
ion because the trifluoromethyl
group is attracting electron
density away from the carboxylate.
The trifluoromethyl
group is an electron
withdrawing group:
 |
 |
|
| Acetate ion | |
Trifluoroacetate ion |
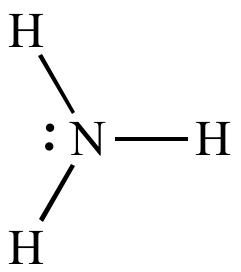
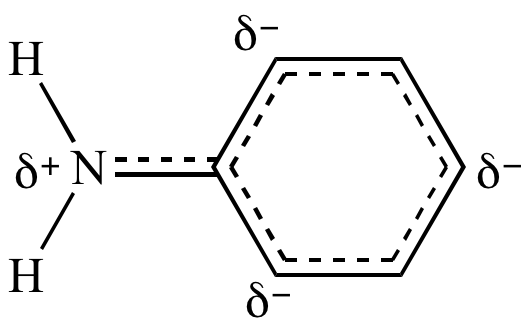
Aniline
is a weaker base
than ammonia
because the benzene
ring is attracting electron
density away from the nitrogen lone
pair by resonance.
The benzene
ring is an electron
withdrawing group:
 |
|
 |
| In ammonia
the lone
pair is localized on the nitrogen atom. |
In aniline,
nitrogen's lone
pair is delocalized
by resonance, as demonstrated by this resonance hybrid. |