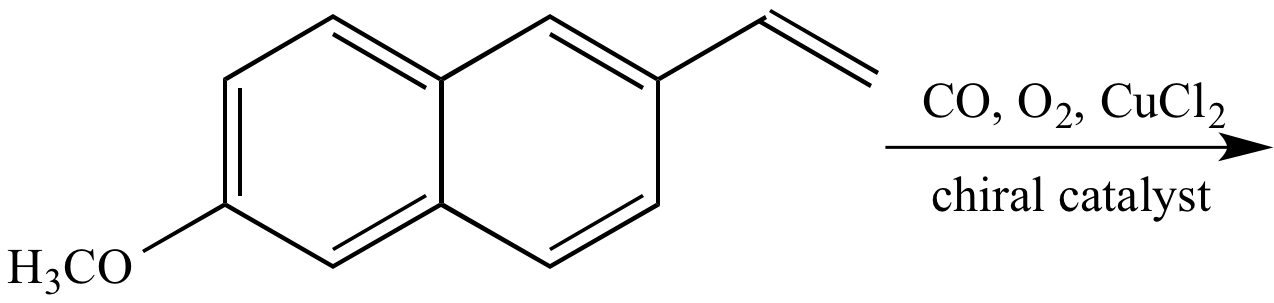
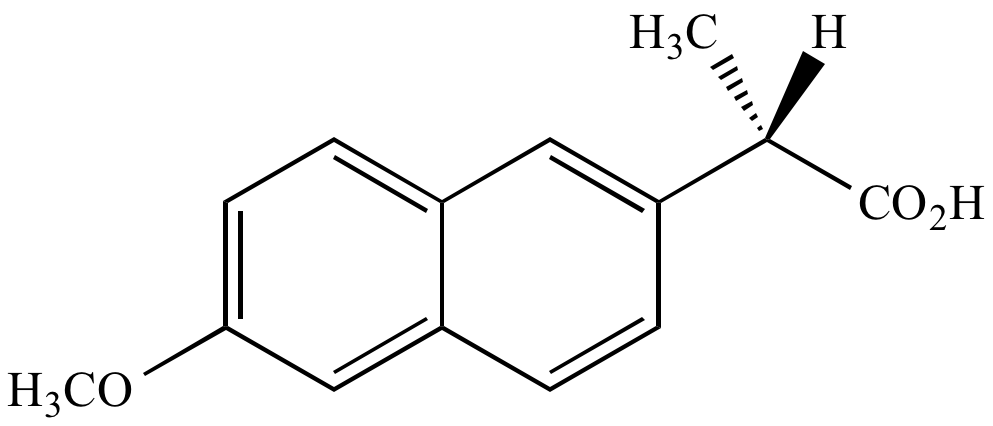
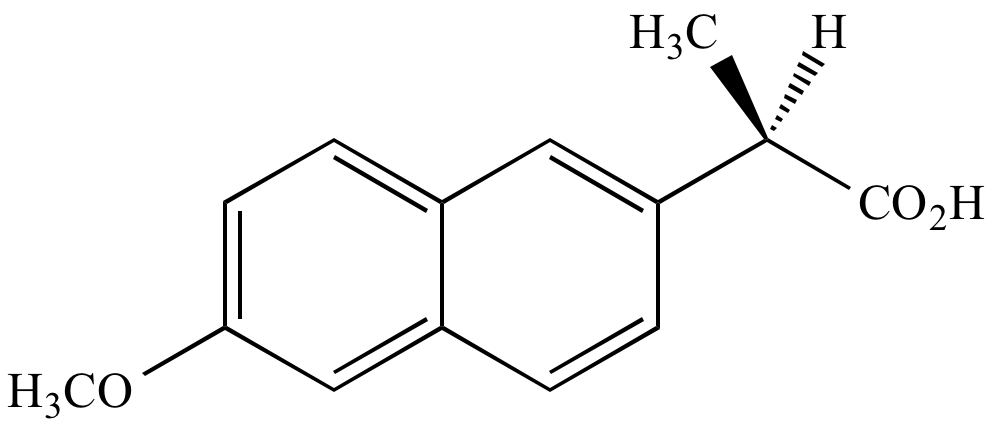
90% of product mixture
Major product
10% of product mixture
Minor product
 |
 |
+ |
 |
| R
enantiomer
90% of product mixture Major product |
S
enantiomer 10% of product mixture Minor product |