 |
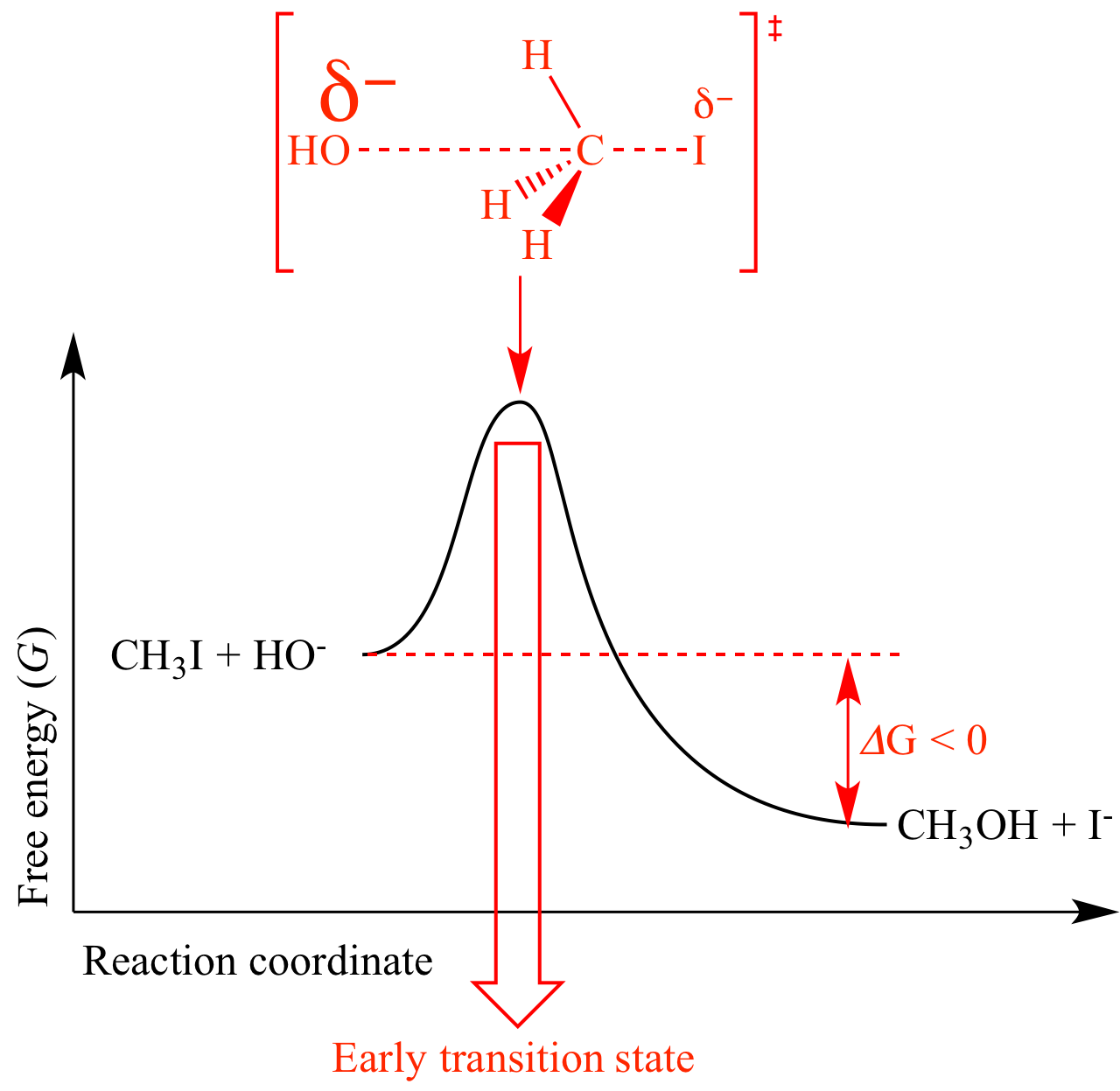
Many SN2
reactions are exergonic
(ΔG
< 0), and therefore have early transition
states. For example, the SN2
reaction of HO-
with CH3I is exergonic
because a carbon-iodine bond
is replaced by a stronger carbon-oxygen bond.
The transition
state resembles HO-
and CH3I (the reactants)
more than it resembles CH3OH
and I- (the products).
In this transition
state the oxygen atom has almost a full negative
charge whereas the iodide atom has only a tiny negative
charge and the carbon-oxygen bond
is very incomplete and the carbon-iodine bond
is almost completely gone. |
|
|
|
|
|||
 |
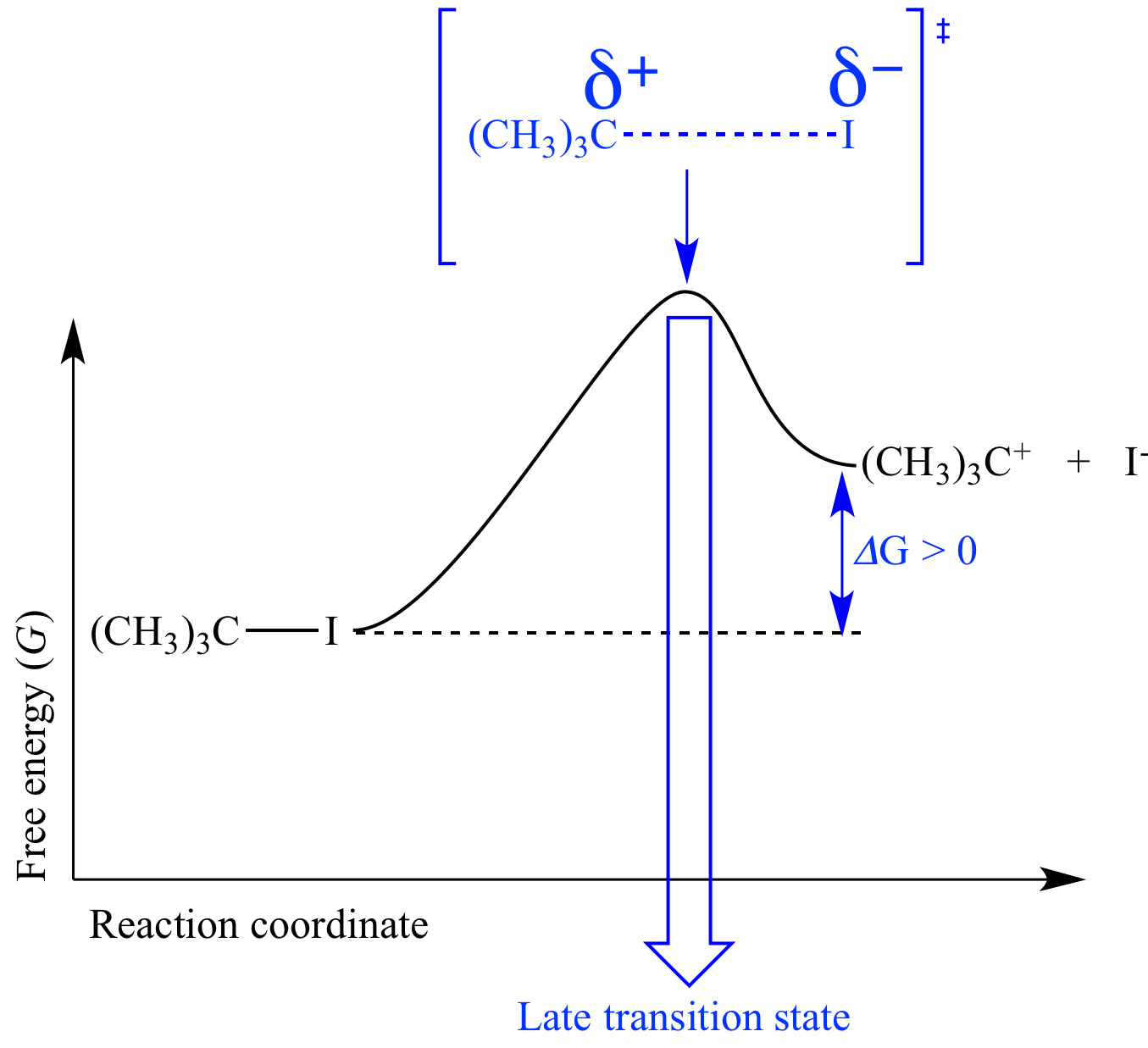
Ionization
of a carbon-leaving
group bond
is endergonic
(ΔG
> 0) because a carbon-leaving
group bond
is lost and no new bond
is formed, and so this mechanism
step has a late transition
state. For example the ionization
of the carbon-iodine bond
of tert-butyl
iodide resembles the tert-butyl
carbocation and iodide ion
(the products)
more than it resembles tert-butyl
iodide (the reactant).
In this transition
state the carbon atom has a nearly full +1 formal
charge and the iodine atom a nearly full -1 formal
charge, and the carbon-iodine bond
is almost completely gone. |