 |
 |
|
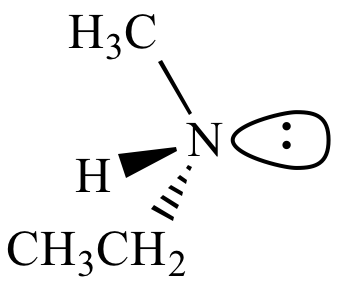
| (S)-Ethylmethylamine |
(R)-Ethylmethylamine |
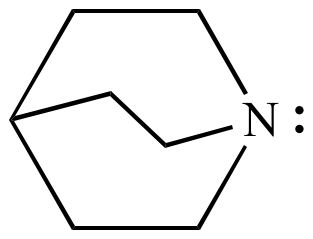
Quinuclidine (shown above) does not undergo nitrogen inversion because the nitrogen inversion transition state structure has too much ring strain. Test this with a model.
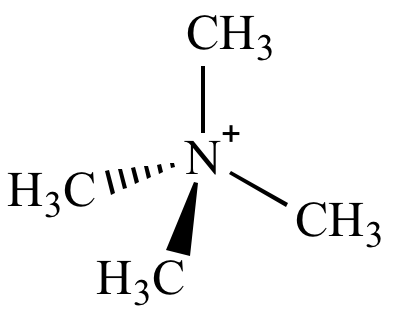
The tetramethylammonium cation (shown above) does not undergo nitrogen inversion because it has no nitrogen lone pair.