One
equivalent:
In reaction stoichiometry,
the amount of one substance that reacts
with one mole
of another substance. This will often (but not always) be a 1:1
mole
ratio.
In this SN2 reaction, one mole of CH3Cl reacts with one mole of NaI to produce one mole of CH3I and one mole of NaCl. One equivalent of CH3Cl reacts with one equivalent of NaI.
| 2 eq.
H2 Pt |
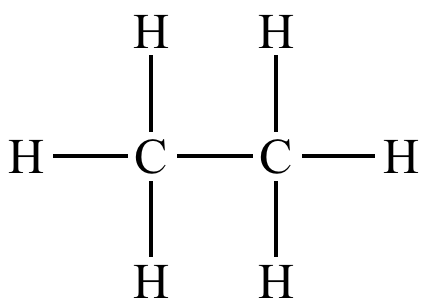 |
| 1 eq.
H2 Pt |
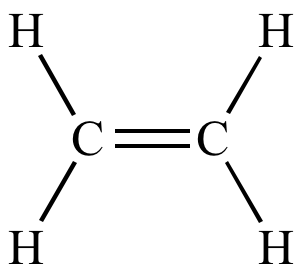 |
1 eq.
H2 Pt |
 |