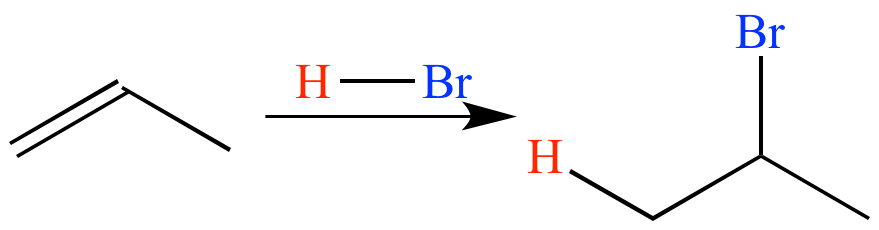
In the absence of a peroxide, HBr adds to propene via an ionic mechanism (with a carbocation intermediate) to give 2-bromopropane. Markovnikov's Rule is obeyed.
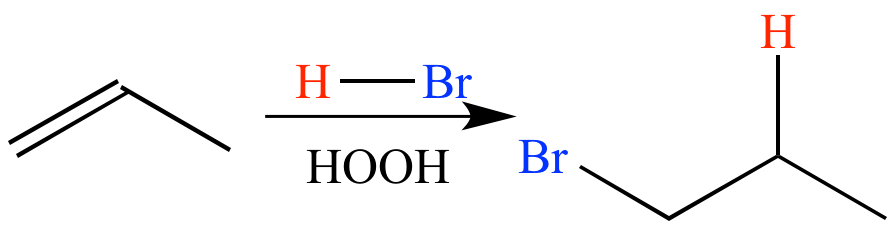
In the presence of a peroxide such as HOOH, HBr adds to propene in an anti-Markovnikov sense and via a radical mechanism, giving 1-bromopropane.