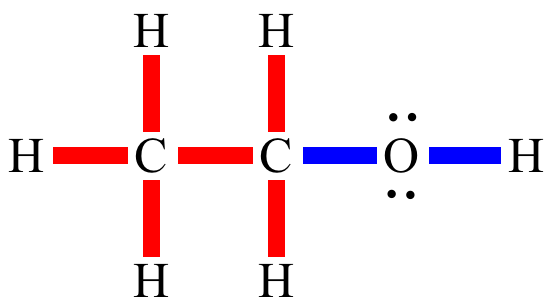
Polar
covalent
bond: A covalent
bond in which the electron
density is unevenly shared between the two bonded
atoms, due to a difference in electronegativity
or due to inductive
effects. In general the electronegativity
difference must be 0.5 or more before the bond
is labeled as a polar
covalent
bond instead of nonpolar
covalent
bond.
|