| Hydrogen 1s orbitals | Hydrogen
molecule
(H2) |
| 2 x |
 |
+ |
6 x |
 |
||
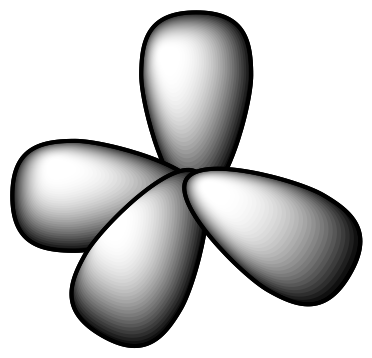
| Carbon sp3
orbitals |
|
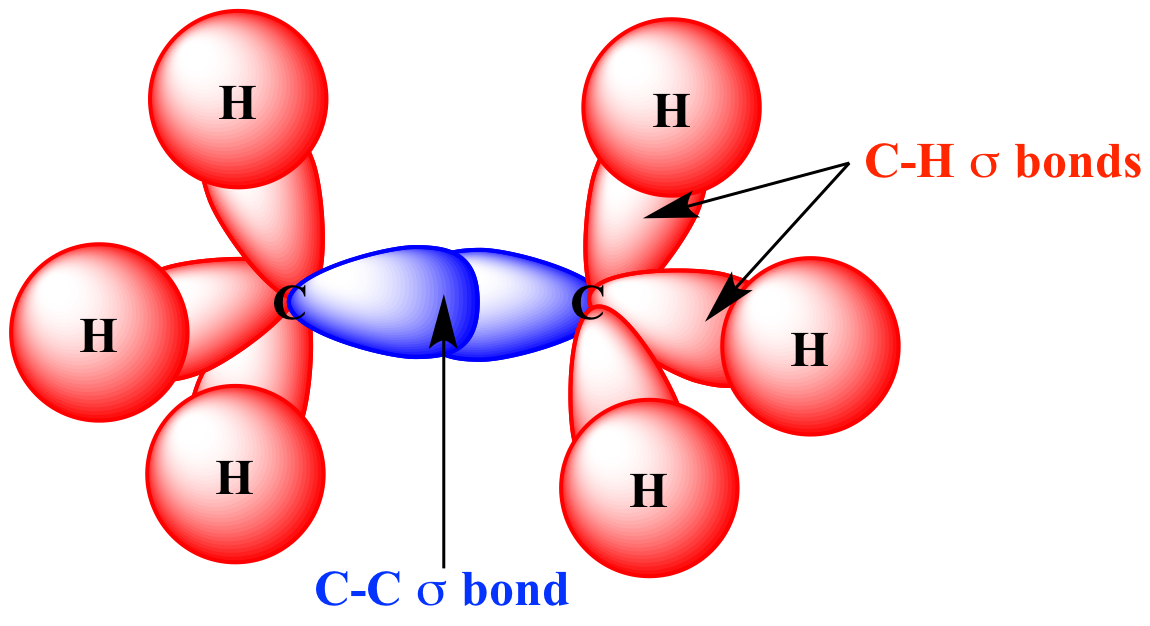
Hydrogen 1s orbitals | C-H
sigma bonds
in ethane
(H3C-CH3) |
|||