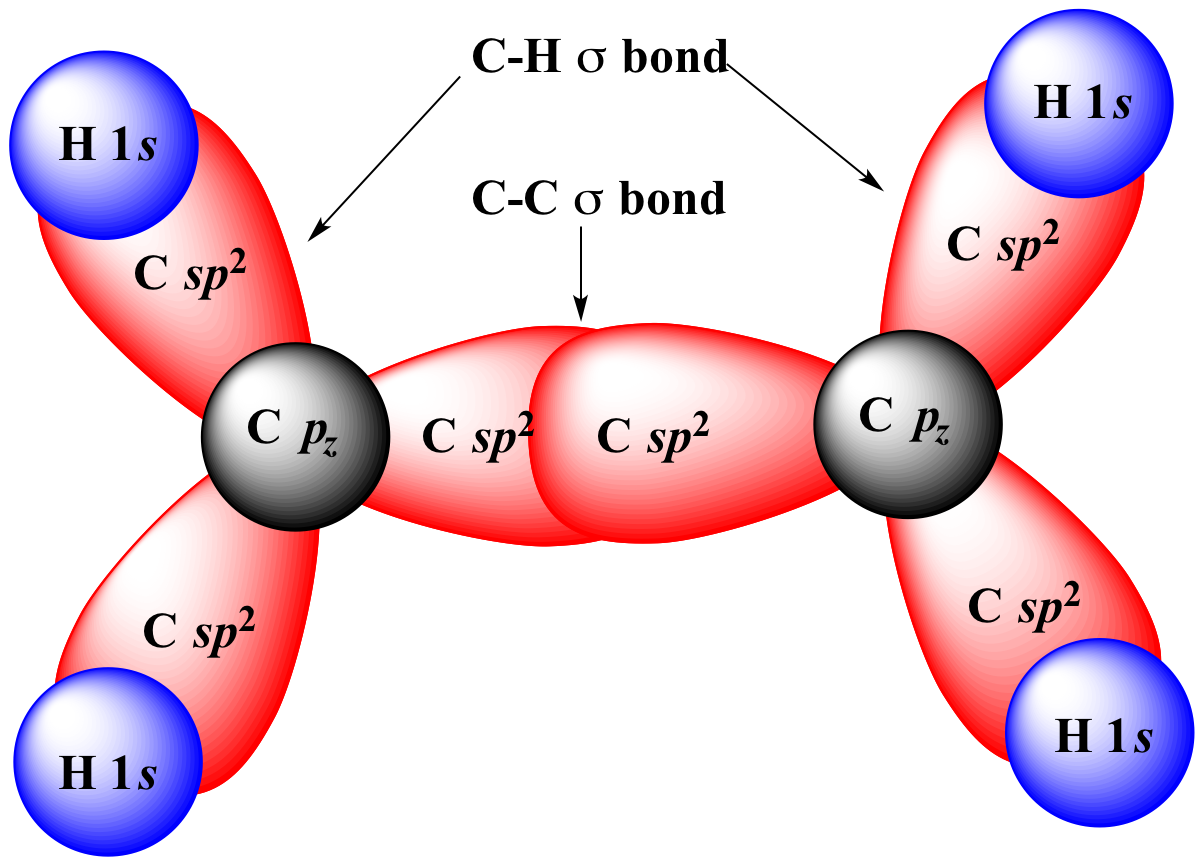
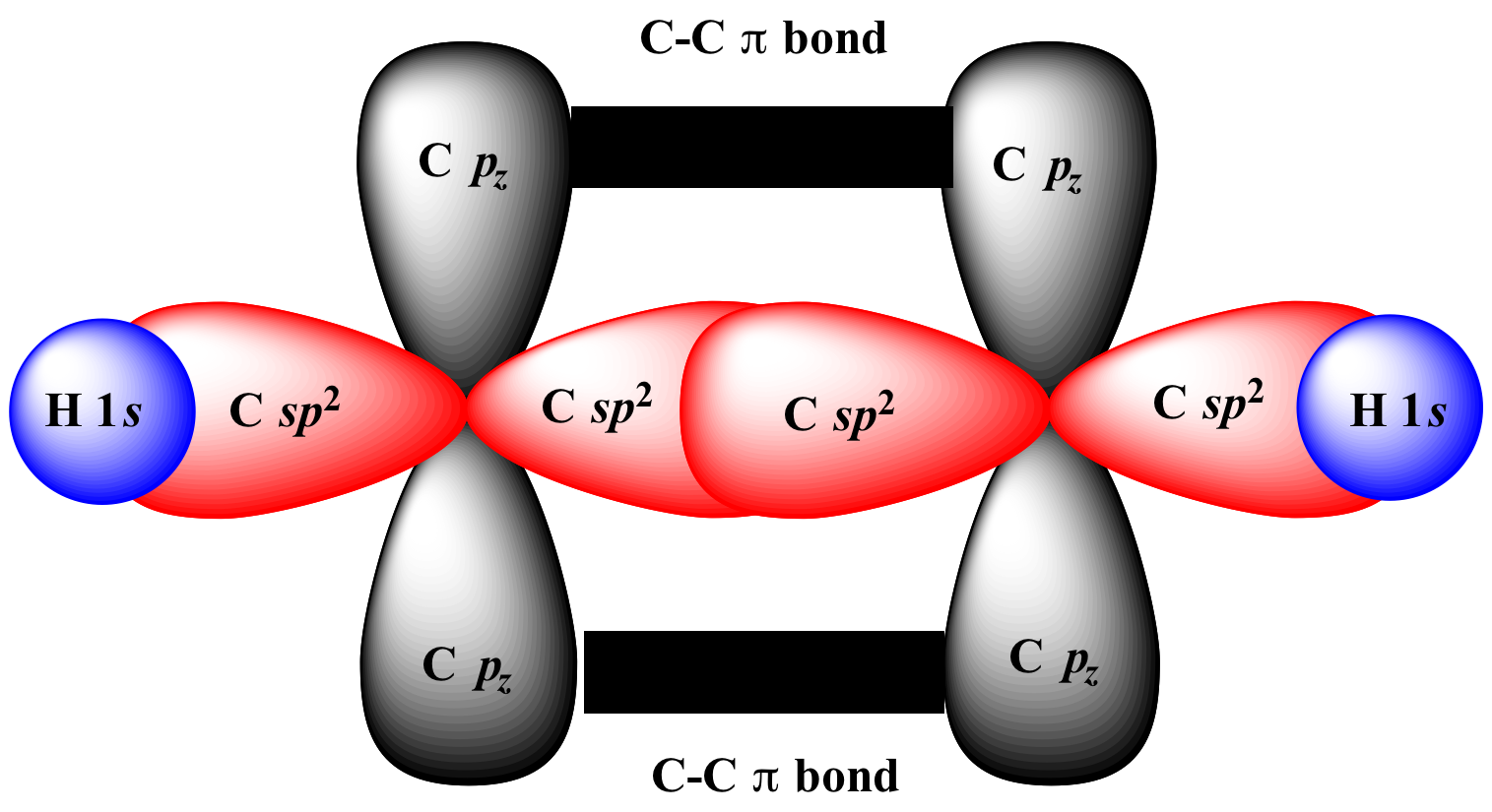
|
+ |
 |
+ |
 |
+ |
 |
merge
to form |
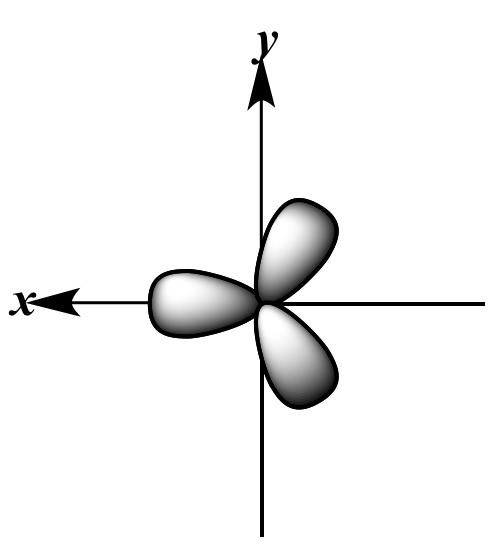 |
+ |
 |
| s orbital |
+ |
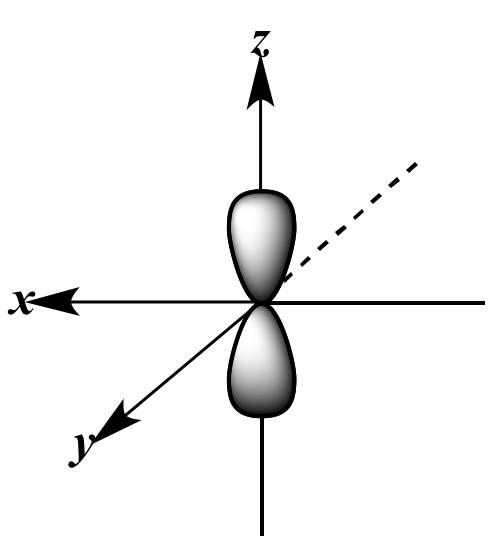
px orbital |
+ |
py orbital |
+ |
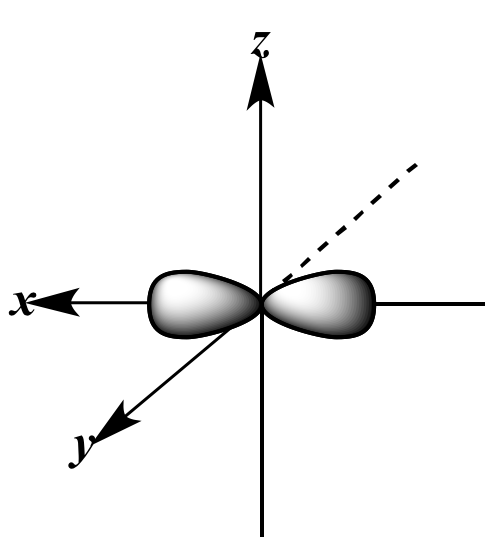
pz orbital |
three sp2 orbitals | + |
pz orbital | |
| Viewed from above the x-y
plane; small back lobes omitted for clarity |