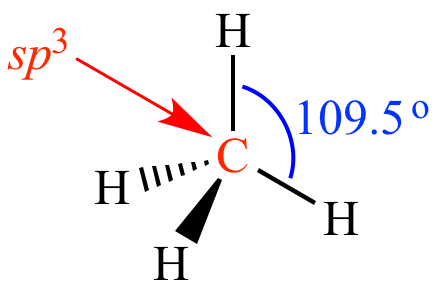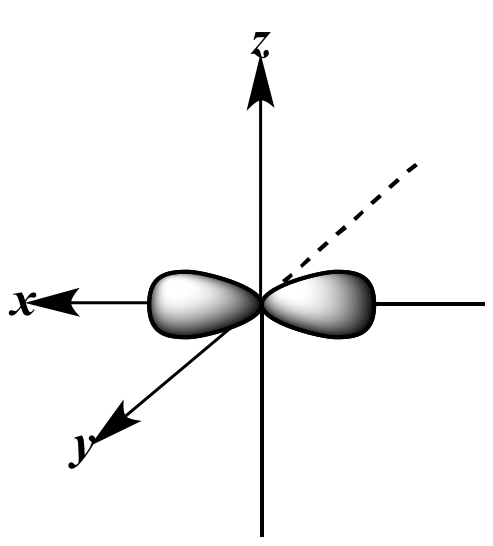

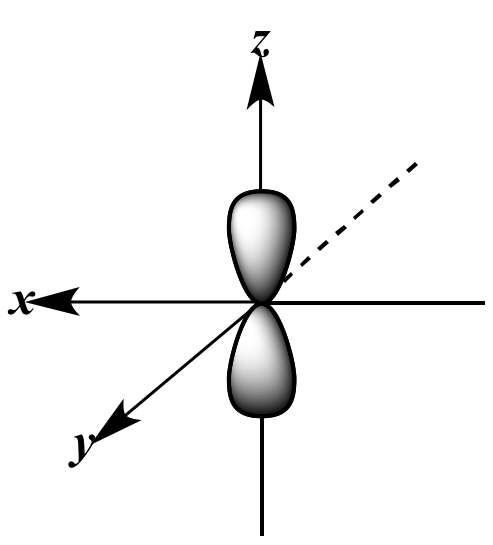
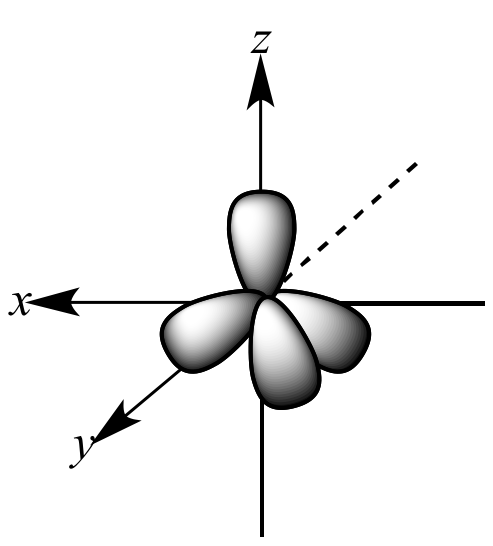
 |
+ |
 |
+ |
 |
+ |
 |
merge to form |
 |
| s
orbital |
px
orbital |
py
orbital |
pz
orbital |
four sp3
orbitals |