| SN2
reaction
rate: |
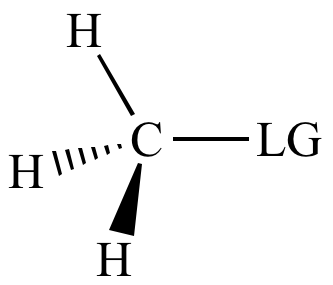 |
> |
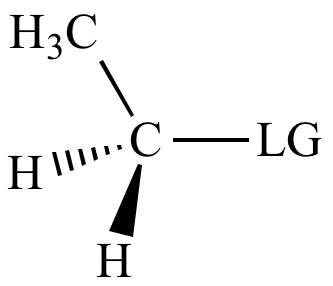 |
> |
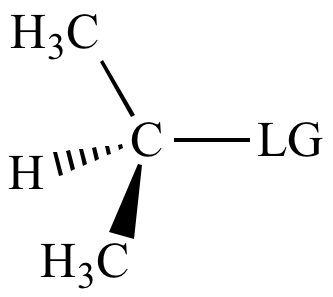 |
>>> |
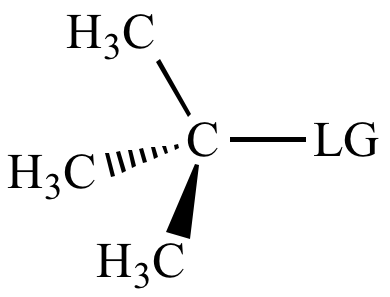 |
| Carbon
bonded
to leaving
group: |
Methyl
carbon |
1o
carbon |
2o
carbon |
3o
carbon |
|||
| Steric hindrance to backside attack: | Least |
Slight |
Modest |
Severe |