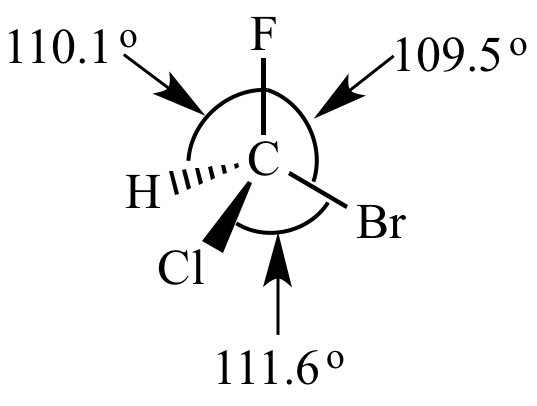
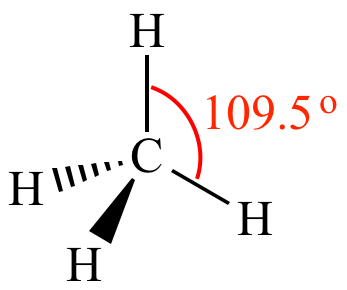 |
 |
 |
All H-C-H bond angles are 109.5o.
The carbon is not a stereocenter.
Build one for yourself to verify
the tetrahedral geometry.
|
|||||||
| Methane All H-C-H bond angles are 109.5o. The carbon is not a stereocenter. |
|
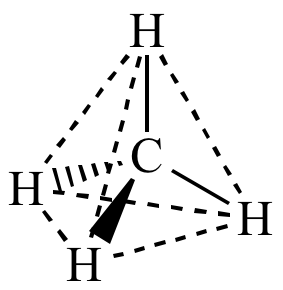
Molecular
model of methane. Build one for yourself to verify the tetrahedral geometry. |