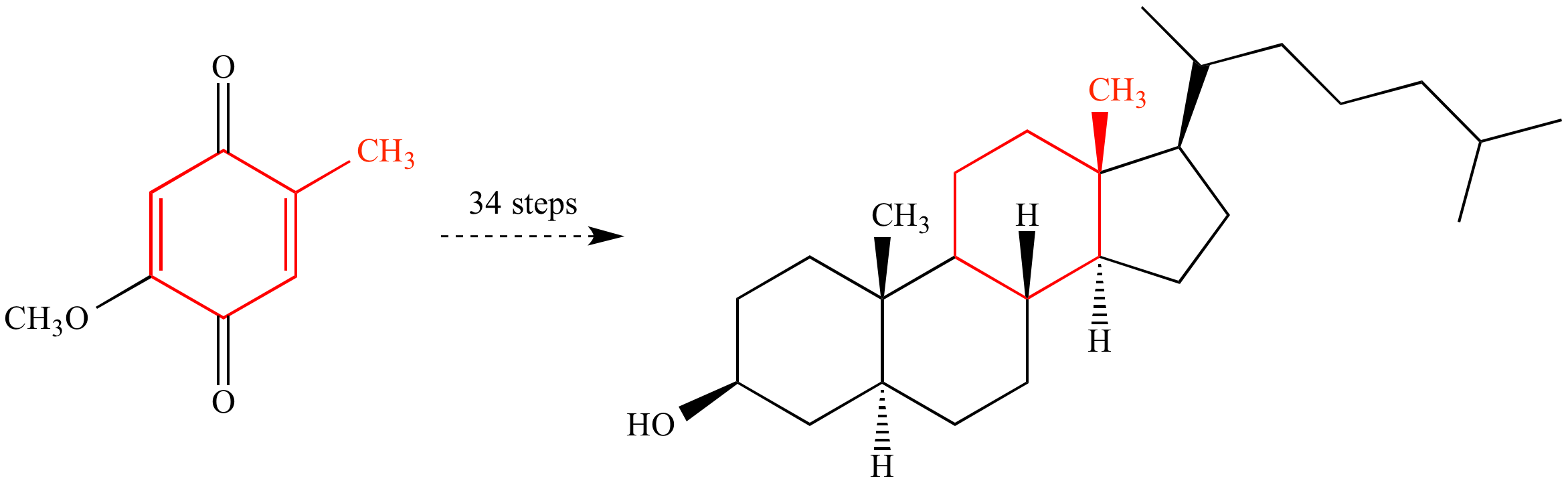
A classic example of total synthesis: Robert Burns Woodward at Harvard University achieved the first total synthesis of cholesterol in 1951. The synthesis required 34 steps (by modern standards, quite inefficient) from a readily-available hydroquinone starting material. The carbon atoms of the starting material that become part of cholesterol are color-coded to show where they appear in the final product.
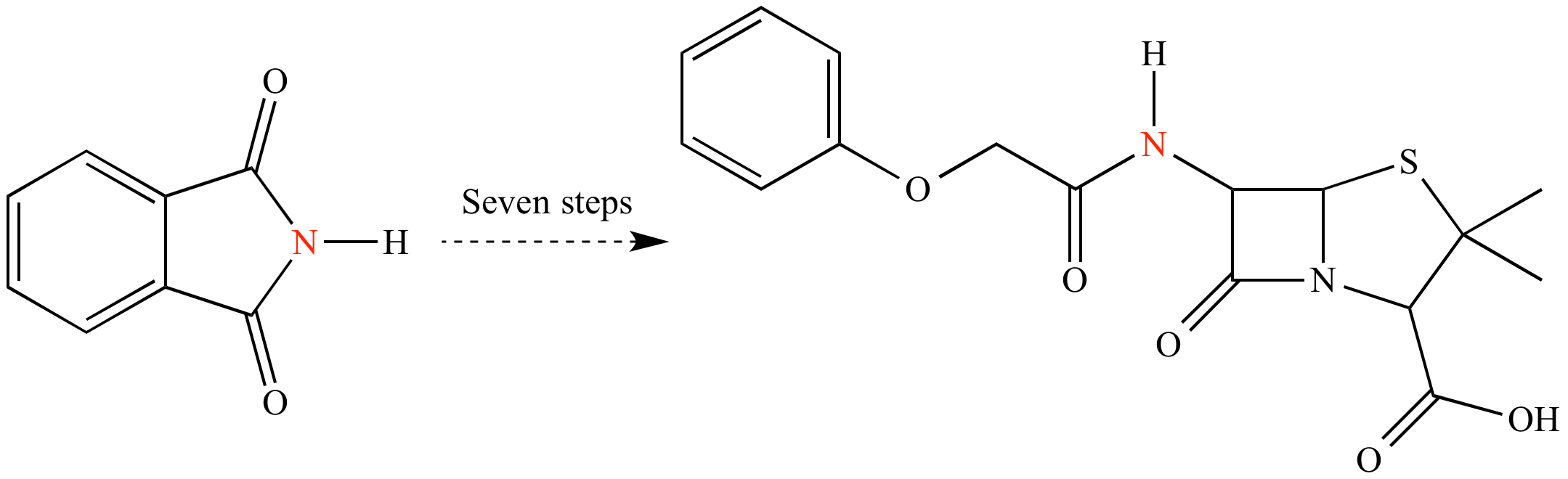
Another classic in total synthesis: Penicillin V, synthesized by John Sheehan at MIT in 1957. The synthesis required a mere seven steps from commercially-available phthalimide. By modern standards, seven steps for a molecule of this complexity is fairly efficient. However the atom economy is poor: Only one atom (the nitrogen; shown in red) of the starting phthalimide ends up in the final target.