updated
Polarimeter
Most physical properties of enantiomers i.e., melting point, boiling point, refractive index, etc. are identical. However, they differ in a property called optical activity, in which a sample rotates the plane of polarization of a polarized light beam passing through. This effect was first discovered in 1808 by E.L. Malus
(1775-1812), who passed light through reflective glass surfaces. Four years later, J.B. Biot
(1774-1862) found that the extent of rotation of the light depends on the thickness of the quartz plates that he used. He also discovered that other compounds i.e.,
turpentine and sucrose solutions were capable of rotating the light. He attributed this "optical activity" to certain features in their molecular structure (asymmetry). Based on his research, he designed one of the first
polariscopes, and formulated the basic quantitative laws of polarimetry. In 1850, Wilhelmy used polarimetry to study the reaction rate of the hydrolysis of sucrose. In 1874, van't Hoff proposed that a tetrahedral environment of the carbon atom could explain the phenomenon of optical activity. Today, polarimetry is used routinely in quality and process control in the pharmaceutical industry,
the flavor, fragrance and essential oil industry, the food industry, and
the chemical industry. The optical purity of the product can be determined by measuring the specific rotation of compounds like amino acids, antibiotics, steroids, vitamins, lemon oil, various sugars, and polymers and comparing them with the reference value (if the specific rotation of the pure enantiomer is known).
|
Compound |
[α]D (in o) |
|
(1R)-(+)-Camphor |
+44.26 |
|
Sucrose |
+66.47 |
|
Cholesterol |
-31.50 |
|
D-(+)-Glucose |
+52.70 |
|
D-(-)-Fructose |
-92.00 |
|
Morphine |
-132.00 |
|
L-Proline (in water) |
-84.00 |
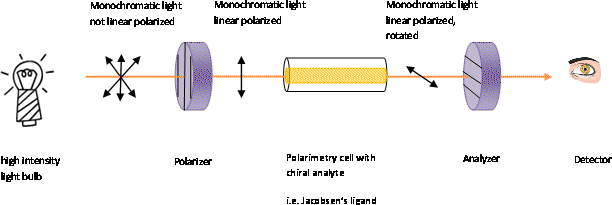
How does it work? Normal monochromatic light contains light that
possesses oscillations of the electrical field in all possible
planes perpendicular to the direction of propagation. When light is
passed through a polarizer (i.e., Nicol prism, Polaroid film) only
light oscillating in one plane will leave the polarizer ("picket
fence model"). This linear polarized light can be described as a
superposition of two counter-rotating components, which propagate
with different velocities in an optical active medium. If one
component interacts stronger than the other with a chiral molecule,
it will slow down and therefore arrive later at the observer. The
result is that the plane of the light appears to be rotated because
the two vectors are not canceling each other anymore due to the
phase shift.

In a polarimeter (figure 2), plane-polarized light is introduced to a tube (typically 10 cm in length, figure 3) containing a solution with the substance to be measured. If the substance is optical inactive, the plane of the polarized light will not change in orientation and the observer will read an angle of [α]= 0o. If the compound in the polarimetry cell was optical active, the plane of the light would be rotated on its way through the tube. The observed rotation is a result of the different components of the plane polarized light interacting differently with the chiral center. In order to observe the maximum brightness, the observer (person or instrument) will have to rotate the axis of the analyzer back, either clockwise or counterclockwise direction depending on the nature of the compound. For clockwise direction, the rotation (in degrees) is defined as positive ("+") and called dextrorotatory (from the Latin: dexter=right). In contrast, the counterclockwise direction is defined as negative ("-") and called levorotatory (from the Latin laevus=left). Unfortunately, there is no direct correlation between the configuration [(D/L) in Rosanoff, (R/S) in Cahn-Ingold-Prelog nomenclature] of an enantiomer and the direction [(+) or (-)] in which they rotate plane-polarized light. This means that the R-enantiomer can exhibit a positive or negative value for the optical rotation depending on the compound. In some cases, the solvent has an impact on the magnitude and the sign as well i.e.,(S)-lactic acid exhibits an optical rotation of
[α]= +3.9o in water and [α]= +13.7o using 2 M sodium hydroxide solution as solvent because the observer looks at a different species (lactate).
The observed specific rotation
[α]obs
depends on the length of the tube, the wavelength that it is used
for the acquisition, the concentration of the optical active
compound (enantiomer), and to a certain degree on the temperature as
well. However, the temperature effect is very difficult to specify
since it differs for each compound. For instance, the
[α]-value for α-pinene only slightly increases in the range from 0 oC to 100 oC (at λ=589.3 nm), while it is almost cut in half for ß-pinene. These two compounds only differ by the position of the alkene function.
Generally the following equation is used to calculate the specific optical rotation from experimental data:
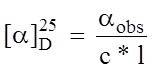
αobs = observed optical rotation
c = the concentration of the solution in grams per milliliter
l = the length of the tube in decimeters (1 dm=10 cm)
Example 1:
A student obtained the following specific optical rotation from his measurement.
![]()
This notation means that the measurement was conducted at 25 oC using the D-line of the sodium lamp (λ=589.3 nm). A sample containing 1.00 g/mL of the compound in a 1 dm tube exhibits an optical rotation of 3.5o in clockwise direction. Note that the instrument used in Chem
30BL and Chem 30CL can provide the specific optical rotation, which already corrects the optical rotation for the cell dimensions and the concentration. The optical rotation is raw data, which does not include these corrections. It is very important to pay attention which mode was used to acquire the data!
As mentioned earlier, polarimetry can be used to determine optical purity of enantiomers.

Example 2:
The observed specific optical rotation of a compound is
[α]= +7.00o. The specific optical rotation for the pure enantiomer is .
| Percent optical purity |
The sample consists of 75 % of the racemic form (=equimolar mixture of both enantiomers, α=0o) and an excess of 25 % of the enantiomer in question (62.5 % and 37.5 %).
The instrument used below allows you to calculate the specific rotation, if you know the concentration of the solution. The cell used for the measurement has a pathlength of 10.0 cm.

Actual polarimeter used in the lab (Autopol IV) located in YH 6104

Polarimetry cell (5 cm stainless steel cell shown here)
Practical Aspects
The cell has to be handled carefully since it costs more than $1000 to manufacture. It has to be cleaned thoroughly after the measurement was performed and is returned to the teaching assistant or instructor. Special attention should be given to the inlets that have been broken off several times already due to negligence on the student’s part!
1. The instrument has to warm up for at least 10-15 minutes, if it is not already turned on. The switch is located in the back of the instrument. The proper wavelength is chosen.
2. A solution with a known concentration (~0.5-3 %) of the compound in the proper solvent is prepared.
3. The polarimetry cell is filled with the solvent. After filling the cell, the path through the cell should be clear (If the path is not clear, the air bubbles in the path have to be removed prior to the measurement). The cell is placed on the rails inside the instrument, all the way on either the right or the left side.
4. The "Zero button" is pressed to zero the instrument. The screen should show 0.000 and not fluctuate too much. If this is not the case, make sure that the light can pass through. If this does not solve the problem, inform the teaching assistant or instructor about this problem immediately.
5. Then, the solvent is removed and the cell is dried. The solution of the compound is filled into the dry polarimeter cell making sure that the entire inner part is filled without any air bubbles or particulate matter.
6. The "I" button on the keypad is pressed and specific rotation is selected.
7. The proper cell dimension is selected: 100 mm (the cell provided is 100.0 mm=1 dm long)
8. Next, the proper concentration in % is entered (=the actual concentration of your solution and not the one recommended since they will most likely differ slightly!)
9. The reading on the display (=specific optical rotation) is recorded including the sign. (The experimenter has to research the literature data before performing the measurement in order to see if he is in the correct ballpark!).
10. The cell is taken out, cleaned thoroughly with the solvent used for the measurement and returned it to your teaching assistant or instructor. If the student is the last one to perform a measurement for the day, the instrument has to be turned off as well.
11. The sample from the optical rotation measurement can be recovered after the measurement if needed by removing the solvent i.e., Jacobsen ligand.
12. It is entirely unacceptable that the student locks up the cells somewhere, where they are not available to others because all the students in the course use the polarimetry cells. Doing so will result is a significant penalty for the student at fault.
Links:
http://rudolphresearch.com/products/polarimeters