
last updated
Introduction
Thin-layer chromatography (TLC) is a very commonly used technique in synthetic chemistry for identifying compounds, determining their purity and following the progress of a reaction. It also permits the optimization of the solvent system for a given separation problem. In comparison with column chromatography, it only requires small quantities of the compound (~ng) and is much faster as well.
Stationary Phase
As stationary phase, a special finely ground matrix (silica gel, alumina, or similar material) is coated on a glass plate, a metal or a plastic film as a thin layer (~0.25 mm). In addition a binder like gypsum is mixed into the stationary phase to make it stick better to the slide. In many cases, a fluorescent powder is mixed into the stationary phase to simplify the visualization later on (e.g. bright green when you expose it to 254 nm UV light).
Preparing the Plate
Do not touch the TLC plate on the side with the white surface. In order to obtain an imaginary start line, make two notches on each side of the TLC plate. You can also draw a thin line with pencil. Do not use pen. Why? The start line should be 0.5-1 cm from the bottom of the plate.

Capillary spotters
Place a melting point capillary and in the dark blue part of the Bunsen burner flame. Hold it there until it softens and starts to sag. Quickly remove the capillary from the flame and pull on both ends to about 2-3 times its original length. If you pull the capillary inside the flame, you will have a "piece of art", but not a good spotter. Allow the capillary to cool down, and then break it in the middle. Make sure that you break off the closed end on one of them. Do not use gloves when you pull capillaries. You will have much better control without them!


Watch movie how to pull capillaries here here
Spotting the plate
The thin end of the spotter is placed in the dilute solution; the solution will rise up in the capillary (capillary forces). Touch the plate briefly at the start line. Allow the solvent to evaporate and spot at the same place again. This way you will get a concentrated and small spot. Try to avoid spotting too much material, because this will deteriorate the quality of the separation considerably (‘tailing’). The spots should be far enough away from the edges and from each other as well. If possible, you should spot the compound or mixture together with the starting materials and possible intermediates on the plate. They will serve as internal reference since every TLC plate is slightly different.

Developing a Plate
A TLC plate can be developed in a beaker or closed jar (see picture below). Place a small amount of solvent (= mobile phase) in the container. The solvent level has to be below the starting line of the TLC, otherwise the spots will dissolve away. The lower edge of the plate is then dipped in a solvent. The solvent (eluent) travels up the matrix by capillarity, moving the components of the samples at various rates because of their different degrees of interaction with the matrix (=stationary phase) and solubility in the developing solvent. Non-polar solvents will force non-polar compounds to the top of the plate, because the compounds dissolve well and do not interact with the polar stationary phase. Allow the solvent to travel up the plate until ~1 cm from the top. Take the plate out and mark the solvent front immediately. Do not allow the solvent to run over the edge of the plate. Next, let the solvent evaporate completely.
  |
 |
 |
 |
|
TLC chamber for development e.g. beacher
with a lid or a closed jar |
after ~5 min | after ~10 min | after drying |
Visualization
|
Reagent |
Works well for |
Colors |
Notes |
|
Iodine |
Unsaturated and aromatic compounds |
Brown
spots |
Not
permanent |
|
Sulfuric acid |
General
stain |
Brown
or black spots |
|
|
Chromic
acid |
For
difficult to stain compounds |
Black
spots |
|
|
UV
light |
Compounds with extended conjugation like aromatic compounds |
Pink on
light green background |
Only
visible under UV light |
|
Cerium
sulfate |
Good
general stain, very well for alkaloids |
|
|
|
Ferric
chloride |
Phenols |
Purple |
|
|
Ninhydrin |
Amino
acids, amines |
Purple |
|
|
2,4-Dinitrophenylhydrazine |
Aldehydes, ketones |
Yellow/orange |
also
called “DNP” |
|
Vanillin |
Good
general stain, very well for hydroxyl or carbonyl compounds |
Colors
vary |
|
|
Potassium permanganate |
Works
well for all compounds that can be oxidized |
Yellow
on purple
|
at r.t.
for alkenes and alkynes upon
heating
for alcohols, amines, sulfides |
|
Bromocresol Green |
Carboxylic acids (pKa<5) |
Yellow
spot on blue background |
|
|
Cerium
molybdate (CAM, ‘Hanessian’s Stain’, Ceric staining) |
Good
general stain, very well with polyhydroxylated and carbonyl
compounds |
Blue or
green spot |
Upon
heating, very sensitive! |
|
p-Anisaldehyde |
Good
general stain, particularly sensitive towards nucleophiles |
Varying
colors on light pink plate upon heating
|
Does not work with alkenes, alkynes or aromatic
system unless functional groups are present |
|
Phosphomolybdic acid (PMA) |
Very
sensitive |
Dark
green spot on light green plate |
Sensitivity can be enhanced by use of cobalt(II) chloride |
|
Ehrlich’s Reagent (Dimethylaminobenzaldehyde) |
Indoles, amines |
Pink or
red-violet |
|
|
Dragendorff-Munier Stain |
Amines
even the ones that are low in reactivity |
Various
colors |
|
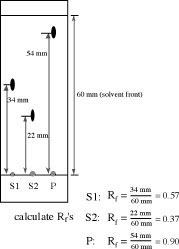
The Rf (=retardation factor) depends on the following parameters:
Due to the fact that all those variables are difficult to keep constant, a reference compound is usually applied to the plate as well.
Useful links