

Pressure adapter for the top of the column consisting of a small glass tube and a thermometer adapter (black or grey)
last updated
The main task for this meeting will be to purify the crude epoxide by column chromatography, and to submit a NMR and GC sample.
General Comments about Column Chromatography
1. It is very important to pack the column properly in order to accomplish the desired separation.The column
cannot have any cracks or air bubbles in the stationary phase. The column should also be clamped properly (=straight!) in order to provide a good separation.
2. Since the stationary phase consists of very small grains, the resulting packing is very dense. In order to ensure a reasonable flow rate of the eluent, pressure has to be applied from the top using
an adapter. This also means that the burette that you check out from lab support has to be in proper condition when you check it out and when you return it!
Setup:
a. Dry Method
Obtain a 25 mL burette that is not be chipped on the top! Clamp it securely in the hood. Place a small cotton ball (~0.5 cm) on the bottom, to cover up the plug from the top. Then add 0.5-1 cm of
sea sand on top of it. Level the sand and then add ~20-25 cm of silica gel (~up to the 7 mL mark). Level the powder and then add another 0.5-1 cm of
sea sand on top. Prepare ~100 mL of the eluent mixture (as determined by TLC) that you plan to use as mobile phase. Carefully fill the column with the eluent to approximately 1 cm from the top. Open the stopcock at the bottom of the column and place a big beaker underneath. Then place a thermometer adapter (or rubber stopper) with a glass tube (has to be checked out from the lab support as well). Since the diameter of the glass tube is usually too small, it has to be increased by applying a small amount of parafilm on the top. Then open the regulator for the air supply slowly. Hold on to the adapter. This will generate a pressure that pushes the solvent through the column faster. When the solvent front is 1 cm away from the sand at the head of the column, take the tubing out and add more solvent. Repeat this procedure until the entire column is wetted. After this happened, allow the solvent level to go down to the sand level.
b. Wet Method (slurry)
Obtain a 25 mL burette, and clamp it securely in the hood. Place a small cotton ball on the bottom. Then add 0.5 cm of sand on top of it. Then pour ~10 mL of the eluent into the column, before you add a slurry of the stationary phase (silica gel) slowly using a powder funnel. Allow the solid to settle down. Make sure that there are no gas bubbles in the column. If this is not the case, add ~0.5 cm of sand on the top. After this happened, allow the solvent level to go down to the sand level.
c. Why two
Methods?
Although the first method is usually faster, many researchers prefer the second method
because it limits the formation of gas pockets and cracks. More polar solvents can react with the stationary phase and virtually start to boil inside the column. This will lead to the formation of gas bubbles, which will deteriorate your separation quality and the elution speed. In either way, the final product should look like the picture below.
 |

Pressure adapter for the top of the column consisting of a small glass tube and a thermometer adapter (black or grey) |
Conditioning
Most silica batches are slightly acidic. Unfortunately, epoxides are very sensitive towards acids. Thus, the acidic residue has to be neutralized first. This is done by applying a 1 % solution of triethylamine in hexane to the column prior to applying the sample. The conditioning step will not be necessary if the compounds are reasonable stable under acidic conditions (i.e., acetylferrocene, ferrocenyl chalcone, etc.).
Running the Column
The crude product is dissolved in 2-3 mL of the eluent (or a non-polar solvent). This mixture is applied carefully to the top of the column. The mixture is pushed into the column using an adapter (consisting of a thermometer adapter with a glass tube with a tight seal (see picture on the right above). The container is rinsed with 2-3 mL of eluent and then loaded onto the column as well. The column is carefully filled with eluent and the air is then used to force it through at a rate of ~10 mL per minute. If the drip rate appears to slow, the teaching assistant or instructor should be consulted for help. A minimum of ten 10 mL fractions are collected in clean test tubes. The collection has to be started as soon as the sample is on the column! It is not uncommon that the student starts to collect later and ends up losing the product because it was combined with waste.
Identification of product-containing Fractions
Upon completion of the column chromatography step, TLC is used again to identify the fractions that contain the desired product. Starting with fraction 3, a TLC is obtained for every other fraction. Three spots can be placed on one plate. If the proper fractions (fraction range) is identified, the fraction before and after the identified fractions are examined as well. The fraction containing the product are combined and the solvent is carefullly removed.
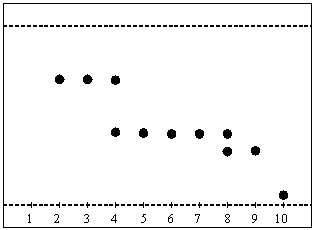
In the diagram above, the fractions 5-7 should be collected as epoxide. Fraction 4 is contaminated with the alkene, while fraction 8 is most likely contaminated with the aldehyde/ketone. Fraction 10 contains a very polar compound i.e., catalyst, diol, etc.
NMR and GC/MS (see reader and lecture for more details)
Upon completion of the flash chromatography step, the final product is isolated and has to be analyzed.
1. A NMR sample of the product is prepared. If enough material is available, ~0.1 mL of the compound is dissolved in 0.5 mL of deuterated chloroform. The NMR tube has to be at least 16 cm in length. The level of teh solution has to be as close as possible to 5 cm in height. This will improve the acquisition process significantly. The solution placed in a clean and dry NMR tube, which is labeled on the top part with a Sharpie (Student Name, Chem 30CL, Section, Unknown X). The sample also has to be signed in on a log-sheet provided by the TA. Proper labeling is important in order to provide the correct NMR spectrum to the student later on.
2. The GC/MS is acquired in a dilute solution (~1-2 mg of epoxide in 1 mL hexane). The sample also has to be signed in on a log-sheet provided by the TA. The same information as above should be included. The GC vial has to be labeled with the unknown!