last updeated
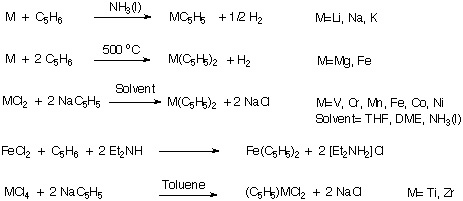
Metallocenes
1. Synthesis

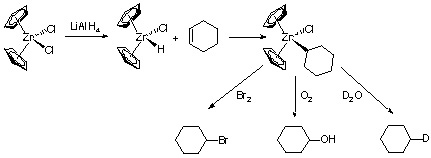
2. Schwartz Reagent
Carbonyl compounds
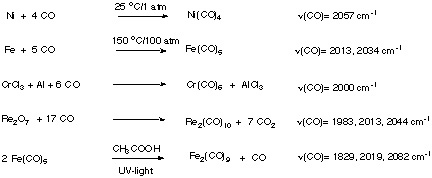
1. Synthesis
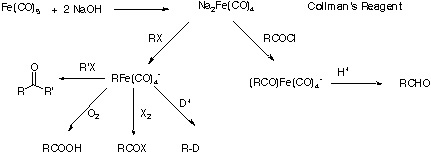
2. Collman's Reagent
3. Structures
1. Nickel tetracarbonyl
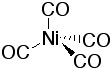 |
Ni(CO)4 forms tetrahedral structure as expected for a d10-system. It is a colorless, highly toxic liquid that boils at 40 oC. Used to purify nickel metal according to the Mond Process (1890) |
2. Iron pentacarbonyl
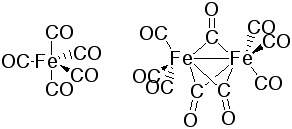 |
Fe(CO)5 forms trigonal bipyramidal structure. It is a yellow, toxic liquid that decomposes in light to form Fe2(CO)9 and CO. Fe2(CO)9 consists of two octahedrons that are sharing one face. Three CO groups are bridging between two iron atoms that are also forming a Fe-Fe-bond. |
3. Dirhenium decacarbonyl
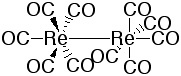 |
Re2(CO)10 forms a white solid. It consists of two octahedrons that are sharing one corner. The CO groups of the neigboring Re-atoms are staggered. The same structure is observed for the Mn and Tc compound. |