|
 |
last updated
Since most reactions do not go to completion or afford byproducts, the crude product is more or less contaminated with other compounds. If the product is a solid, recrystallization is common way to purify the crude product. The main problem is to find a good solvent for this task.
1. Finding a good solvent
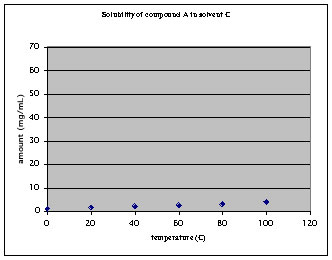
a. The compound displays relatively low solubility at all temperatures
|
 |
In order to dissolve 100 mg of the compound, 25 mL of solvent are required at 100 oC. Upon cooling to 0 oC, 75 mg of the compound will precipitate and 25 mg will stay in solution. Conclusions for this solvent:
1. 25 % of the compound will be lost in solution, 75 % of the compound recovered as precipitate. Not a good recovery!
2. A relatively large amount of solvent was used which raises the cost for this purification step significantly. In addition, a lot of waste is produced as well!
b The compound displays a high solubility at all temperatures
|
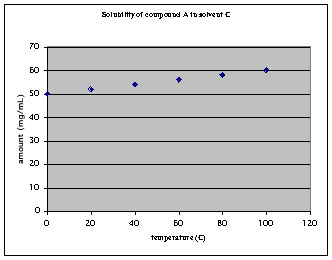 |
In order to dissolve 100 mg of the compound, ~1.7 mL of solvent are required at 100 oC. Upon cooling to 0 oC, 17 mg of the compound will precipitate and 85 mg stay in solution. Conclusions for this solvent:
1. Approximately 85 % of the compound stays in solution and will be lost, only 15 mg are recovered. That is a a VERY poor recovery rate.
2. The solvent quantity is much lower because the overall solubility of the compound is much higher, but due to the low slope of the curve, the recovery is very poor.
c. The compound displays a high solubility at high temperature and a low solubility at low temperature.
|
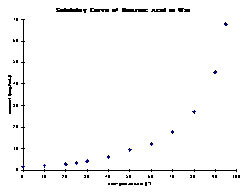 |
In order to dissolve 100 mg of benzoic acid, ~1.5 mL of water are needed at 95 oC. Upon cooling to 0 oC, 97.5 mg of benzoic acid precipitate and 2.5 mg stay in solution. Conclusions:
1. The amount of solvent required is relatively small, which saves costs
2. The majority of the purified sample is recovered (here: 97.5 %) which is highly desirable
d. Solvent Mixtures
Although in theory there always should be a good single solvent to perform a recrystallization, the question is also if it is readily available and reasonably priced. In many cases, one can also resource to a mixture of two solvent in order to accomplish the same task. The boiling points of these two solvents should be very similar and the polarity difference not too extreme to avoid phase separation if a compound is added. Of course, the two solvents have to be miscible in the first place and possess different polarities. The table below shows commonly used solvent mixtures.
| More-polar solvent | bp.(C) | Less-polar solvent | bp.(C) |
| Benzene |
80
|
Cyclohexane |
81
|
| Ethanol |
78
|
Hexane |
69
|
| Ethyl acetate |
77
|
Cyclohexane |
81
|
| Ethanol |
78
|
Acetone |
56
|
| Chloroform |
61
|
Petroleum ether | ~50 |
| Water |
100
|
Ethanol |
78
|
| Water |
|
Acetone |
56
|
However, the solvent mixture should not be boiled excessively to prevent a significant change in composition, which can cause problems during the dissolution or the precipitation part of the procedure.
2. Procedure
There are different ways described how to perform a recrystallization. Which one is the right one for you depends on certain factors i/e. quantity, amount of solvent required, equipment available for the task and last but not least how similar the compounds are.
a. The compound in question dissolves and impurity does not
Dissolve the the compound and remove the impurity by filtration. Strictly speaking, this is not a recrystallization, much more of an extraction.
b. The impurity dissolves and the compound in question does not
Suspend the crude product in a suitable solvent (like-dissolves-like) and filter the mixture afterwards. Strictly speaking, this is not really a recrystallization, much more of an extraction.
c. Both compounds are somewhat similar in the solubility
The key to a pure sample here is to really dissolve everything. This should be done in a suitable solvent (as determined in part 1) and at high temperature (boiling point of the solvent). In many cases, it takes some time to dissolve all the crude product. A watch glass with some ice cubes on top of the Erlenmeyer flask allows you to gently reflux the mixture (less solvent evaporates away during the heating phase). Do not forget to add a boiling stick, boiling stone or a spin bar (that of course should spin) while heating.
The resulting mixture can be filtered hot if necessary with a short stem funnel, and then allowed to cool down slowly in order to obtain clean crystals. The faster the precipitate forms, the more impurties are usually trapped in the solid. A slow crystallization allows the compound to arrange its molecules properly in the solid phase and leave the impurties in solution.
3. Trouble shooting a recrystallization
In case the compound does not crystallize again from solution there might be several things that you can do:
| Problem | Question | Fix |
| Samples does not dissolve | Right solvent or solvent mixture (correct ratio if mixed solvent?) | Double-check solvent |
| Did you heat the mixture to a boil to dissolve the sample? | If not, then you probably will have to much solvent. Boil a significant amount off. | |
| Sample does not crystallize | Did you use a too much solvent (see above)? | If so, boil some of the solvent off |
| Solution is supersaturated | Use a seed crystal or scratch the insides of the flask with a glass rod. |
4. Addition materials
a. See Survival Kit reader: Recrystallization, Vacuum filtration