The yield calculation that you perform for most synthetic procedures is based on the comparision of moles of product isolated and moles of product that you can theoretically obtain based on the the limiting reagent.
For that let's look at one simple example. A student reacts 35.0 g acetic acid and 100 mL of ethanol in the presence of 2 mL of concentrated sulfuric acid. She obtains 44 g of the crude ester and 39.5 g after purification (distillation).
Step 1: Write balanced equation
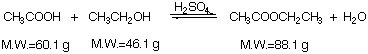
Step 2: Identify limitiing reagent
| Compound | Calculation | Moles of X |
| Acetic acid | 35 g/(60.1 g/mol) | 0.58 mol |
| Ethanol | (100 mL*0.816 g/mL)/(46.1 g/mol) | 1.77 mol |
| Sulfuric acid | (2 mL *1.84 g/mL)/98.04 g/mol | 0.038 mol |
Based on the numbers in the 3rd column, one would think that sulfuric acid is the limiting reagent. Wrong! Why? Please remember the function of the sulfuric acid in this experiment. It is the catalyst to speed up the reaction and does not appear as a part of the product. Keep in mind that catalysts are usually used in 1-10 mol% quantities to speed up reactions and not in stoichiometric quantities.
But back to our calculation problem. After we eliminated the sulfuric acid as limiting reagent, the next higher number is the one of acetic acid. Is this molecule part of the product? Yes, it is indeed. We identified our limiting reagent. Hurray!
Step 3: Calculate the yield
a. How many moles of the product did we isolate?
Crude: 44 g/(88.1 g/mol)= 0.50 mol
Final: 39.5 g/(88.1 g/mol)=0.45 mol
b. Yields
General: Yield = (actual number of moles/theoretical number of moles)*100%
Crude = (0.50 mol/0.58 mol) * 100% = 86.2 % ~86%
Final = (0.45 mol/0.58 mol) * 100% = 77.0 % ~77 %
Note: It does not make any sense to report 10 significant figures at this point. Why?
Before we leave this subject, please keep in mind that most of the reactions that you are going to carry out are performed on microscale. The losses due to transfers are significant on this scale. Most of the reactions carried out in the laboratory run in 80-90% yield on macroscale. If you isolate 60%+, you usually did a pretty good job. The losses are usually more significant for liquids than for solids.