

updated
Fluorescence was first observed about 550 years ago in the extracts
of wood of certain trees. This phenomenon is observed in minerals (i.e.,
aragonite (CaCO3),
calcite (CaCO3),
rubies, emeralds, diamonds), biological systems (i.e., certain
scorpions, fish, parrots, flowers) and organic chemicals (i.e.,
anthracene in toluene). Note that fluorescence is
also found outside the lab in tonic water (blue to the presence of
quinine, see below), in highlighters (pyranine), in bank notes
(security feature), etc.
Fundamentally speaking, fluorescence is the emission of light by a substance that has previously absorbed electromagnetic radiation. In order to excite an electron from the ground state (S0) to the excited state (S1), a certain amount of energy is required (purple arrow below). The excited molecule can subsequently relax by various pathways. In a non-radiative relaxation, the energy is dissipated as heat in form of vibrational energy to the solvent (red arrows). Due to the lower remaining energy of the electron, the emitted light displays a longer wavelength than the absorbed radiation (green arrow). Depending on the intensity of the absorbed light and the quantum yield, the fluorescence can be more or less intense.


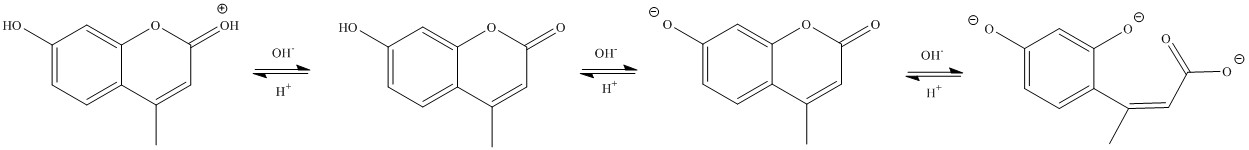
In Chem 30BL, the students will look at the
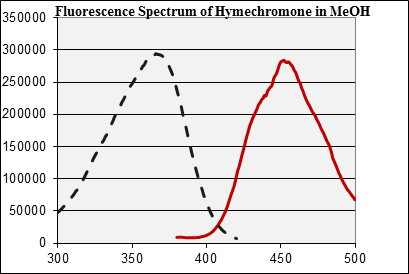
fluorescence of hymecromone. The spectrum below on the right (solid red
line) is the emission spectrum of hymecromone in methanol for the
excitation at λ=365 nm.2 Note that the peak maximum is
at λ=445 nm, which means that there is a 80 nm Stokes' shift.
Going backwards from this peak maximum, one can acquire the excitation
spectrum (dashed black line), which shows that the used wavelength of λ
=365 nm is very close of the excitation maximum. Note that the
correlating maxima in the spectra are very close in intensity, which
means that most of the excited photons are emitted during the emission.
The quantum yield Φ is very high (=photons emitted/ photons
absorbed) in this case. Thus, if the compound is excited using the long
wavelength of the UV-lamp, it
will appear bluish to the observer. If the student would use different
wavelength, the emission would be weaker.
2.