last updated Thu, Apr 10, 2003
In order to speed up reactions or to increase the solubility of a compound, chemists often times reflux reaction mixtures. This step implies that the reaction mixture is brought to a boil. The lowest boiling compound in the mixture determines the temperature when this occurs, usually the solvent. It condenses and returns into the reaction vessel.
The picture below shows a reflux setup for a macro-scale experiment used in upper division laboratories e.g. Chem 144 and Chem 174. It consists of a round bottom flask, a reflux condenser (water-jacketed) and heating source (here: heating mantle). The entire setup is connected to an inert gas line since the used starting material or products are air sensitive in this case.

Even though the micro-scale setup is a little smaller, it still contains similar components: a conical vial (or small round bottom flask), a condenser (air condenser or water jacketed depending which course you are enrolled) and a heating source (most of the time a hotplate/Al-block).
Independent of the scale that you run the reaction, keep the following things in mind:
1. Dimension
Make sure that the reaction vessel is not more than half filled to allow enough room for expansion and boiling.
2. Clamps
The entire setup has to be clamped at the neck of the flask (using a proper sized clamp) or at the air condenser in micro-scale using compression caps. It has to hold the entire weight of the entire setup.
3. Cooling
a. Micro-scale
If you use an air condenser, you will have to cool the condenser using a wet paper towel.
b. Macro-scale
The inlet of the water is on the lower end of the condenser, the outlet at the upper end independent if the condenser is used for reflux or distillation. This way you will require a lower flow rate for cooling, because the outer jacket of the condenser will fill up with water first before it reaches the outlet at the top.
4. Heating
a. Micro-scale
Usually a hotplate is used together with an Al-block. Make sure that the vial or flask fits decently in one of the holes. If it fits too tightly, it might get stuck upon heating. If you don't have an Al-block, you can manufacture a pouch from Al-foil which often does the same job.
b. Macro-scale
The heating mantle should fit the flask size. A heating mantle that is either too small or too big, has a poor contact with the flask, which damages the heating mantle. In addition, the temperature is very difficult to control. The lack of contact causes the heating mantle to overheat and burn out. A heating mantle that is slightly to big can be filled with sand to improve the heat transfer. Most heating mantles have no temperature/power control. An external device has to be attached to control the power input. Never plug the heating mantle directly into a wall plug!!
6. General Warning
If you heat a system, you need to have an 'overpressure valve' somewhere in your setup. It doesn't matter what you heat, the media always will expand (more or less) and will ultimatively build up a significant pressure, which can cause heavy explosions, followed by a fire if you heat flammable or pyrophoric materials.
7. Joints
For a better seal e.g. to perform vacuum distillations, the ground glass joints should be lightly lubricated. The high vacuum grease is usually stable up to ~150 C.
8. Other important issues
Don't forget to add a spin vane, boiling stone or a spin bar to your flask in order to agitate the solution during the reaction/reflux.
9. Recrystallization
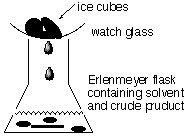
A simple reflux setup for a recrystallization consists of an Erlenmeyer flask with an watch glass with some ice cubes on the top (see below). The solvent will boil and condense at the cold watch glass and drip back into the Erlenmeyer flask.