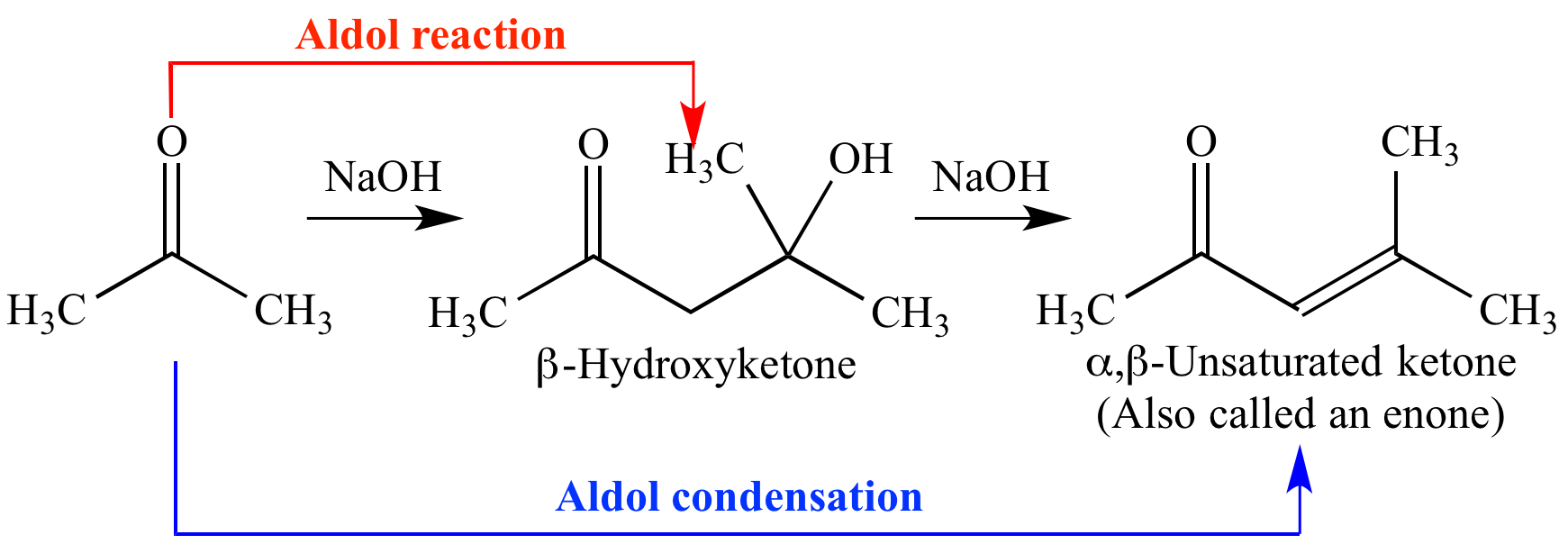
A typical aldol condensation reaction, in which acetone enolate is the nucleophile and another molecule of acetone is the electrophile.
| + |
 |
 |
+ |
 |
+ |
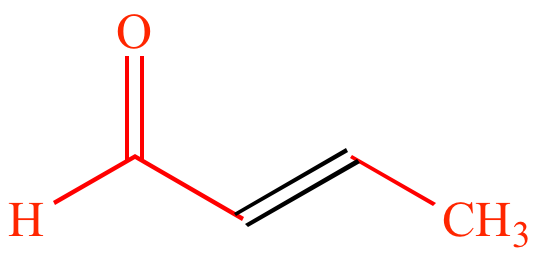 |
+ |
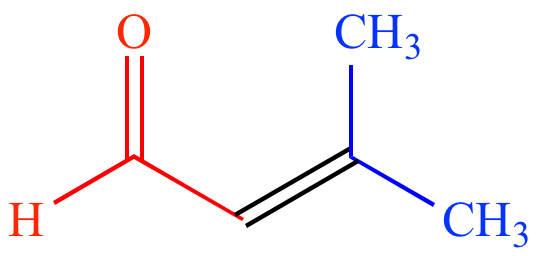 |
||
| Acetone |
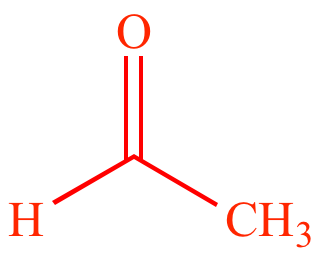
Acetaldehyde |
Nuc
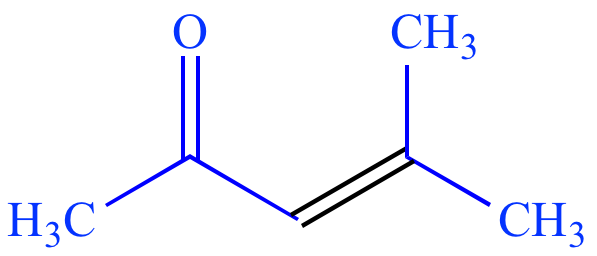
= acetone
enolate Elec = acetone |
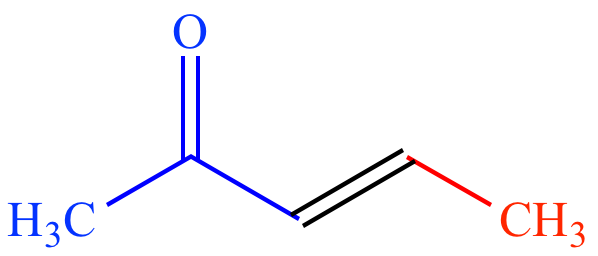
Nuc
= acetone
enolate Elec = acetaldehyde |
Nuc
= acetaldehyde
enolate Elec = acetaldehyde |
Nuc
= acetaldehyde
enolate Elec = acetone |
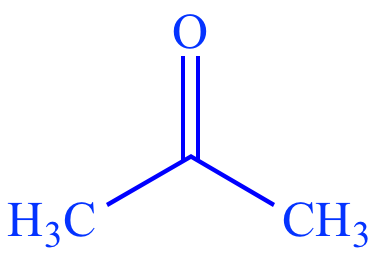 |
+ |
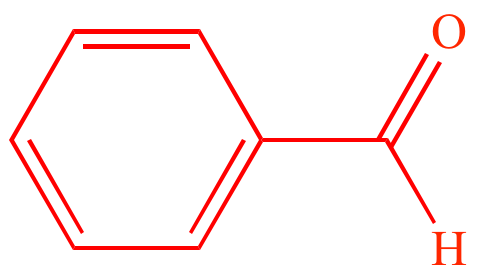 |
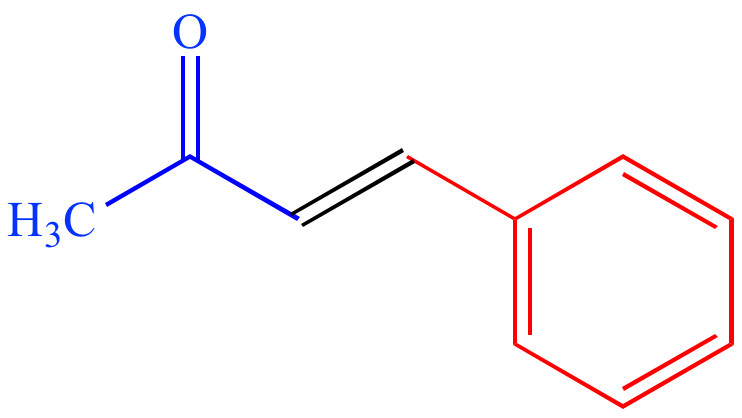 |
|
| Acetone | Benzaldehyde | (E)-4-phenylbut-3-en-2-one |