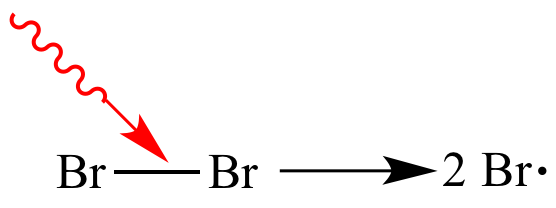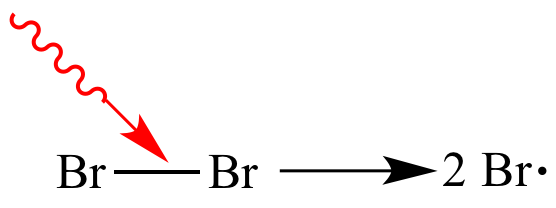|
Arrow
|
Explanation
|
Example
|
|
|

|
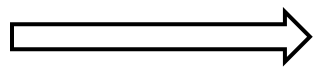
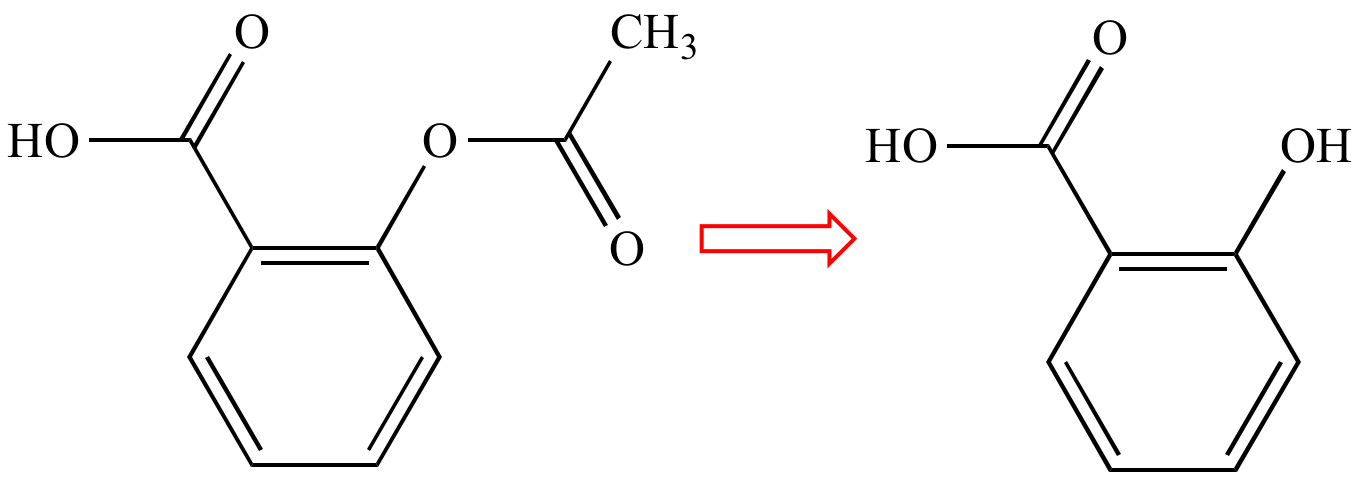
Reaction proceeds from reactants
(on left) to products
(on right). |
 |
|
|
|
|
|
|
|
 |
Indicates electron
donation from a Lewis
base to a Lewis
acid in a Lewis
acid-Lewis
base
adduct: Lewis
base→Lewis
acid. This usage not common. |

|
|
|
|
|
|
|
|
 |
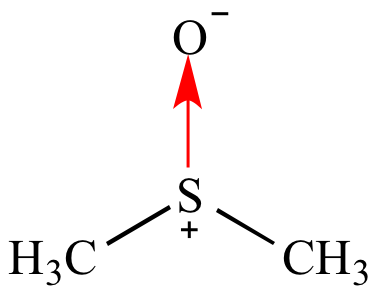
Indicates a pi
bond interaction between elements not in the same period
(row) of the periodic table; a very polar
pi
bond. For example S→O in a sulfoxide.
The arrows points from the less electronegative
element to the more electronegative
element. This usage is not common. |
|
|
|
|
|
|
|
|
|
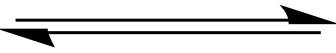
Equilibrium.
Shafts lengths are not meant to imply Keq ≠
1, or stated otherwise. |

|
|
|
|
|
|
|
|

|
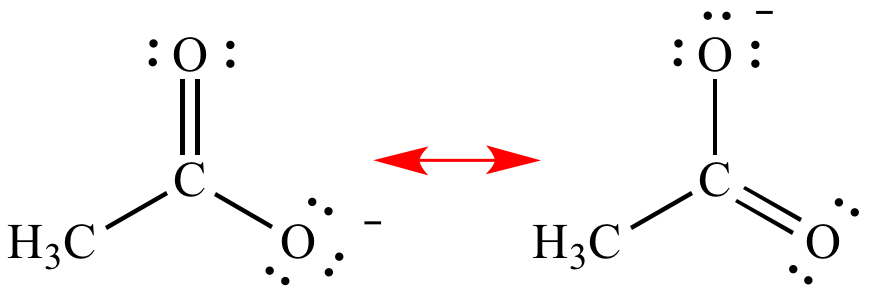
The structures
on either end of the arrow are resonance
contributors. |
|
|
|
|
|
|
|
|

|
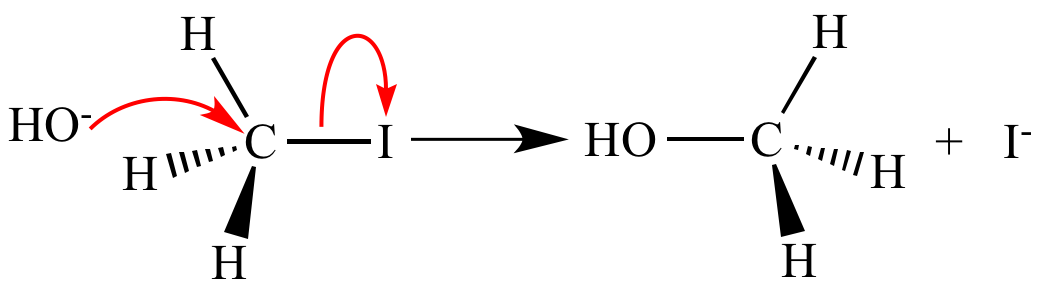
Curved
arrow indicating shift of an electron pair. Note this
arrow has two barbs on the pointy end. |
|
|
|
|
|
|
|
|

|
Curved
arrow indicating shift of a single electron. Note this
arrow has one barb on the pointy end. |

|
|
|
|
|
|
|
|

|
Photon
added. |
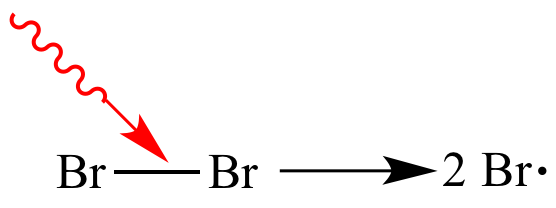
|
|
|
|
|
|
|
|
|
Retrosynthesis
arrow. Designates that the species of the left is derived
from the species on the right. |
|
|
|
|
|
|
|
|
|
Bond
dipole arrow. The arrow head points to the more electronegative
atom (the δ-
atom); the crossed end of the arrow lies above the more electropositive
atom (the δ+
atom). |
|
|
|
|
|
|
|
|

|
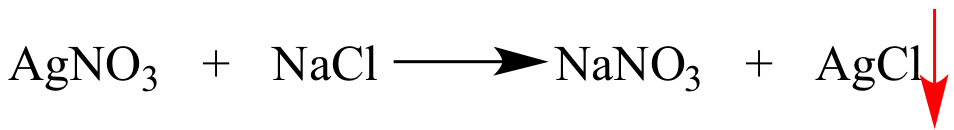
The species to the left of
this arrow leaves the reaction mixture as a precipitate. |
|
|
|
|
|
|
|
|
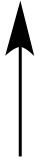
|
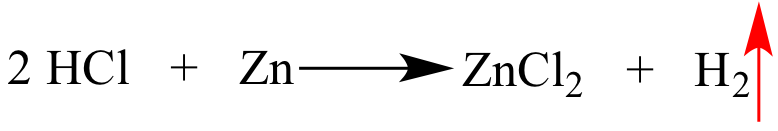
The species to the left of
this arrow leaves the reaction mixture as a gas. |
|
|
|
|
|
|
|
|
|
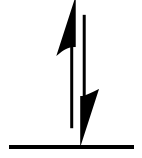
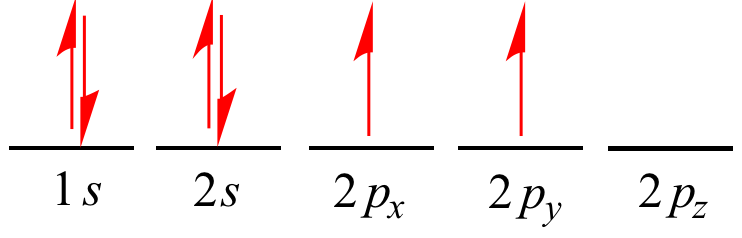
A electron pair in an orbital.
A single arrow (pointing up or down) can be used to denote a
single electron in an orbital.
|
|
|