 |
||||||
| Lewis
structure |
|
Ball and spoke |
|
Space-filling
model |
|
Molecular
model
kit |
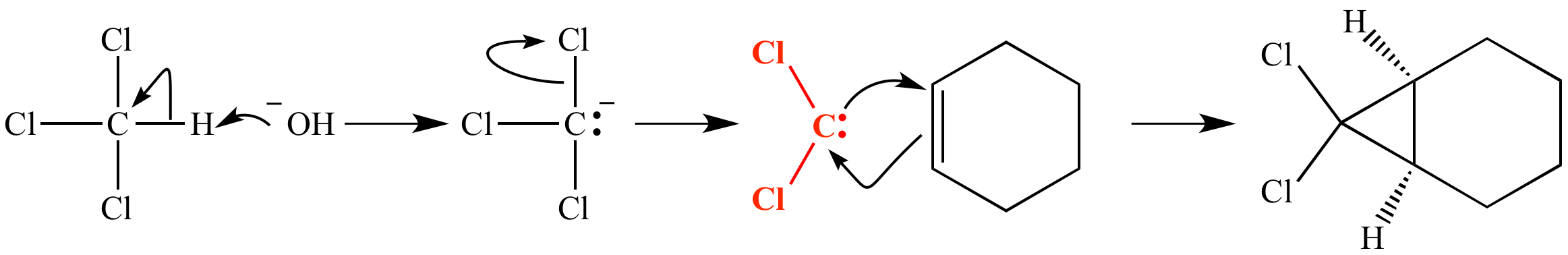
Deprotonation of chloroform with hydroxide ion (a strong base) gives a carbanion. This carbanion ejects chloride ion to give dichlorocarbene, :CCl2 (shown in red). This carbene reacts with an alkene to form a cyclopropane.