six neutrons, resulting in an atomic mass of 12 amu.
seven neutrons, resulting in an atomic mass of 13 amu.
eight neutrons, resulting in an atomic mass of 14 amu.
 |
 |
 |
||
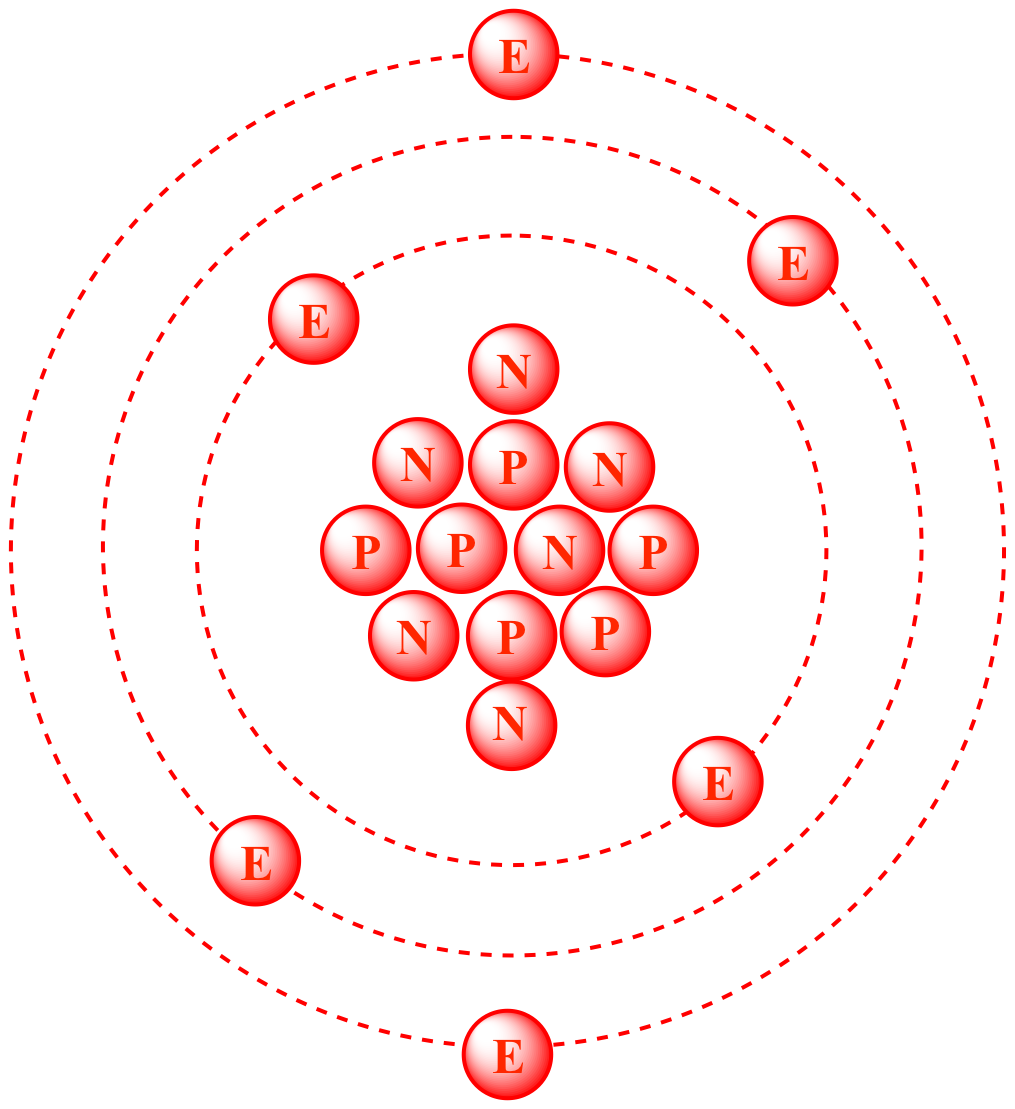
| The nucleus of carbon-12
contains six protons
and six neutrons, resulting in an atomic mass of 12 amu. |
|
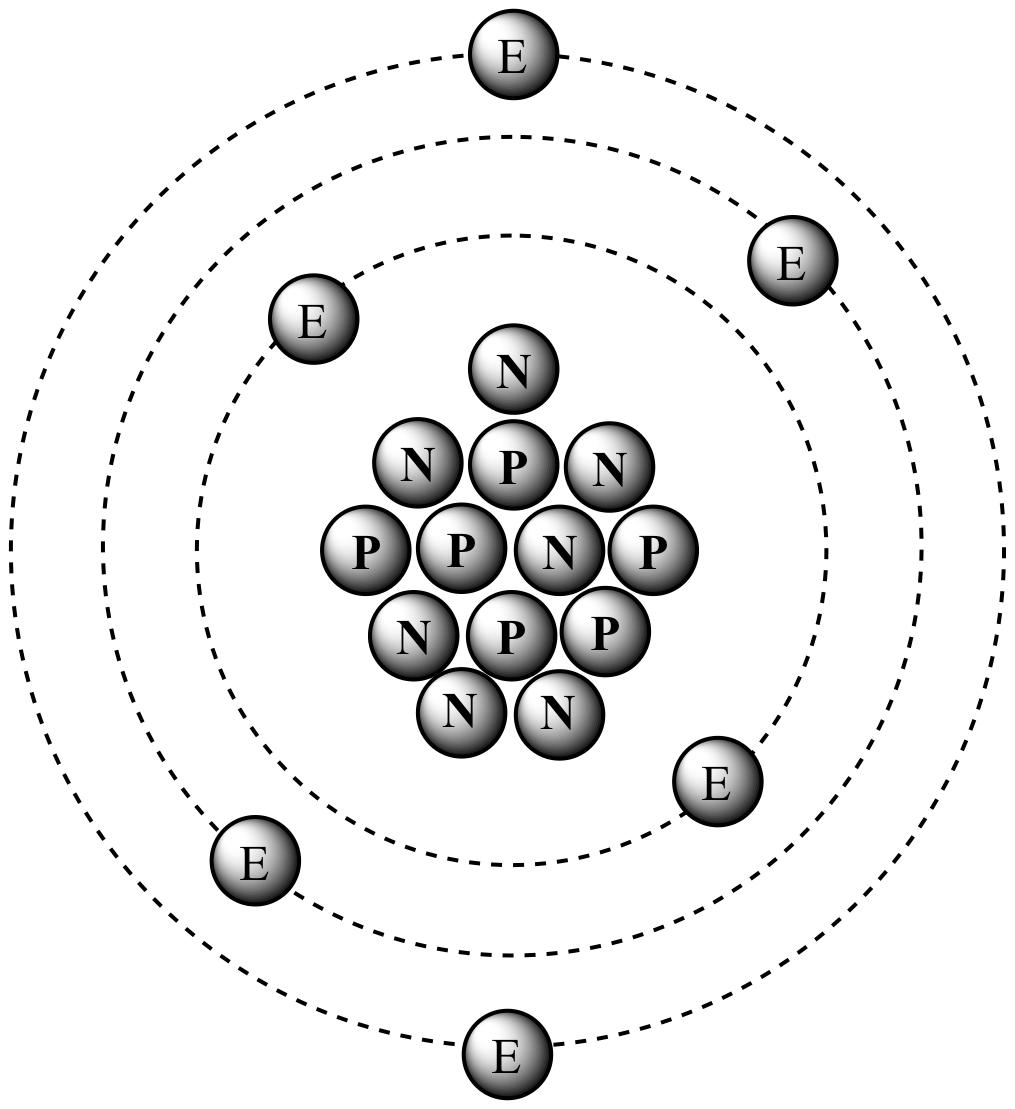
The nucleus of carbon-13
contains six protons
and seven neutrons, resulting in an atomic mass of 13 amu. |
|
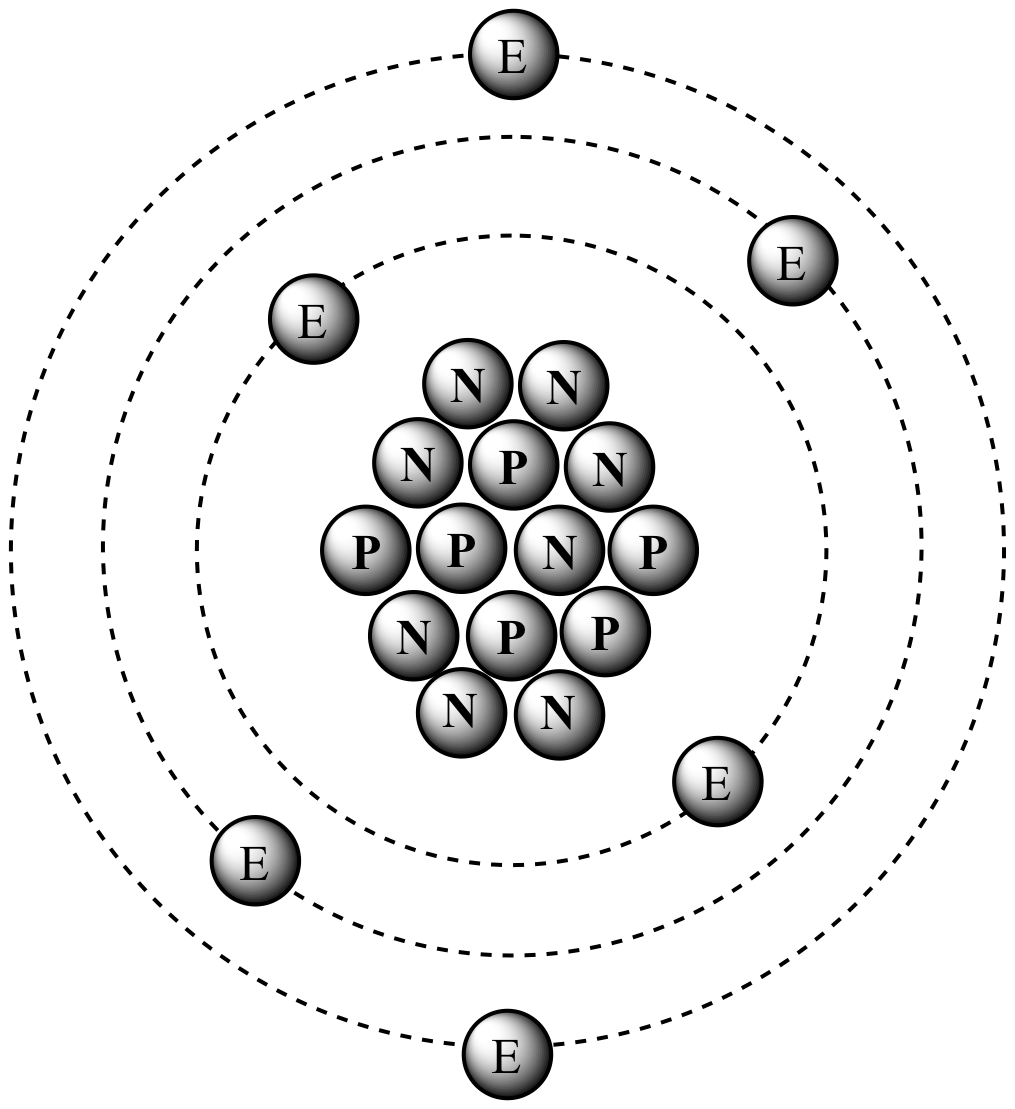
The nucleus of carbon-14
contains six protons
and eight neutrons, resulting in an atomic mass of 14 amu. |