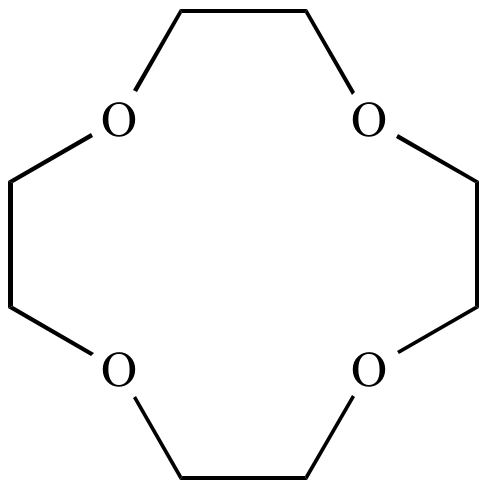
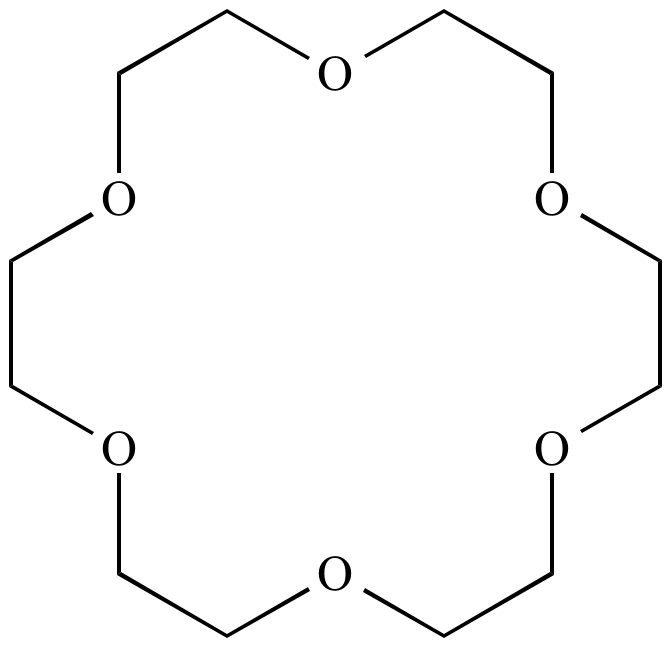
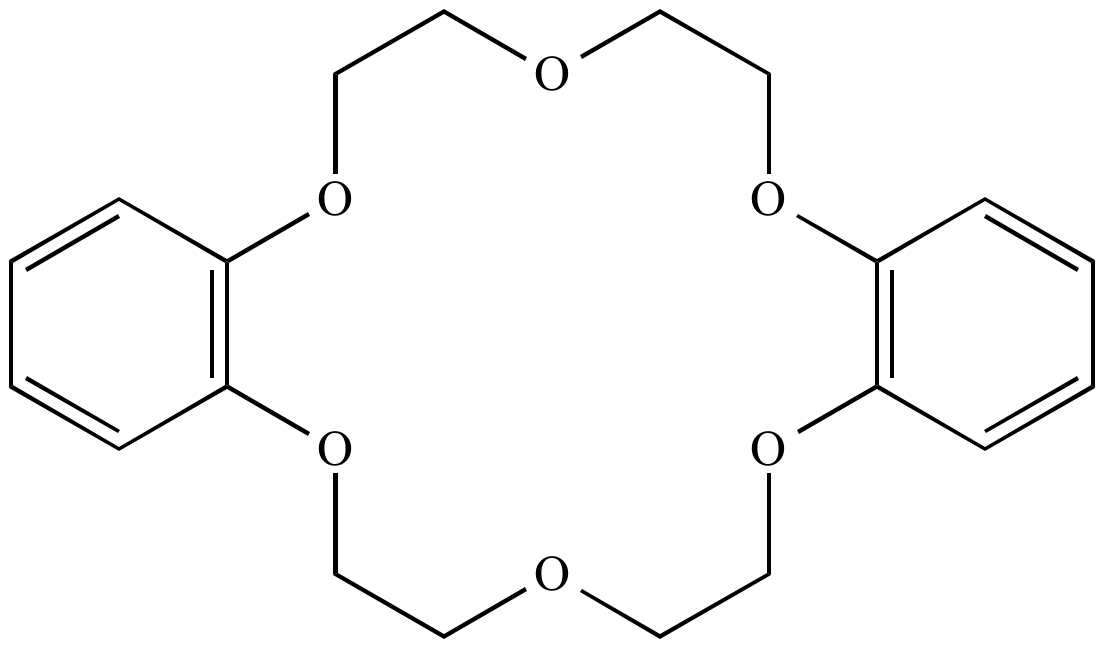
Crown ether:
A series of
cyclic
ethers,
featuring several oxygen atoms each separated by two carbon atoms.
Called 'crown'
ethers
because their three-dimensional shape resembles a crown. Named as
X-crown-Y, where X is the total number of atoms in the ring and Y
is the total number of oxygen atoms. Crown
ethers
are notable because the exhibit selective
cation
binding (i.e.,
molecular
recognition). For example, 12-crown-4 binds Li
+
more strongly than K
+, whereas 18-crown-6 binds K
+
more strongly than Li
+.