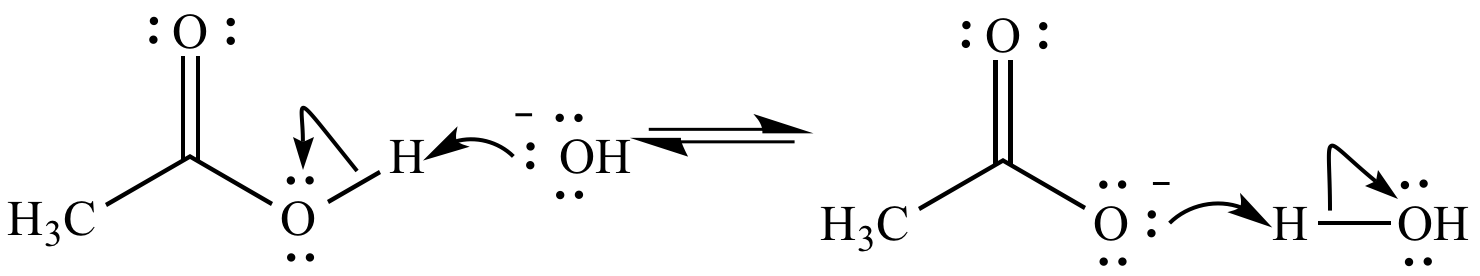
Hydroxide ion (HO-; a Bronsted base) deprotonates acetic acid (CH3COOH; a Bronsted acid) as the reaction moves to the right. (We can also say acetic acid protonates hydroxide ion.) Acetate ion (CH3CO2-) deprotonates water as the reaction moves to the left.