 |
 |
|
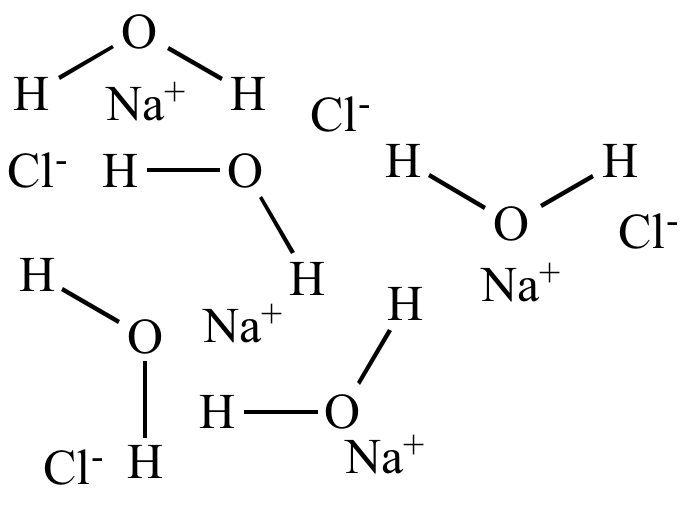
| Water
(ε = 80) High polarity Polar solvent |
|
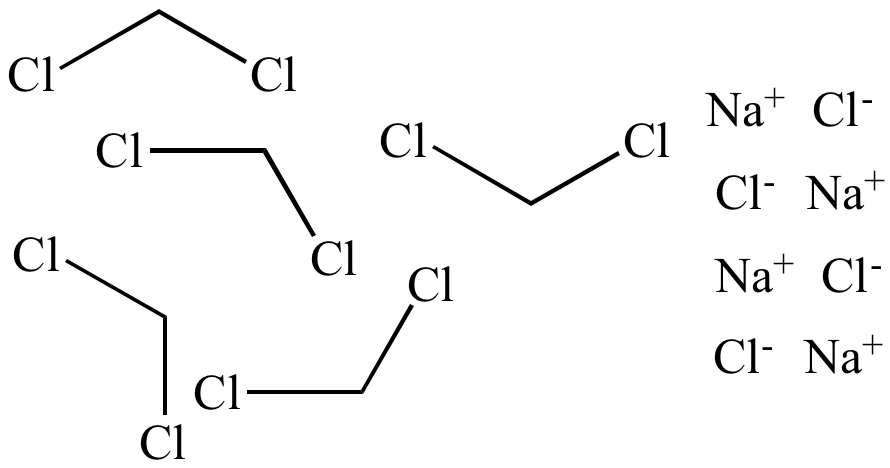
Dichloromethane
(ε = 9.1) Low polarity Nonpolar solvent |
Dielectric Constants of Common Solvents
Polar solvents (ε > 20)
 |
 |
 |
||||||||
| Water ε = 80 |
|
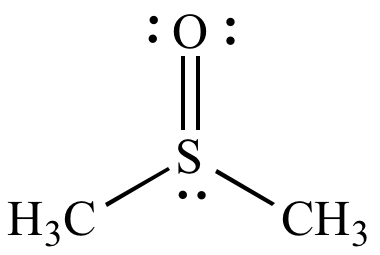
Dimethylsulfoxide (DMSO) ε = 49 |
|
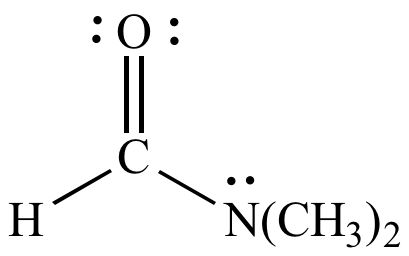
N,N-Dimethylformamide (DMF) ε = 37 |
|
Methanol ε = 33 |
|
Ethanol ε = 25 |
|
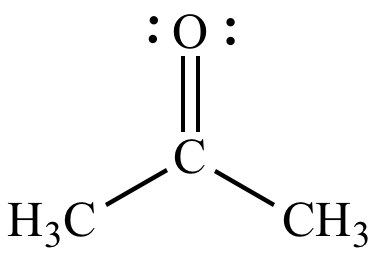
Acetone (propanone) ε = 21 |
Nonpolar solvents (ε < 20)
 |
 |
|||||||
| Dichloromethane ε = 9.1 |
|
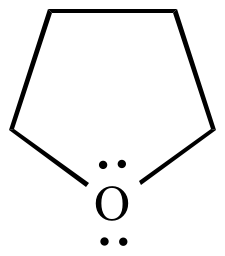
Tetrahydrofuran (THF) ε = 7.6 |
|
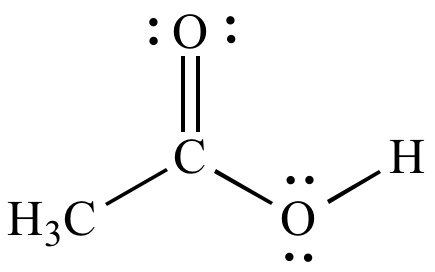
Acetic
acid (HOAc) ε = 6.2 |
|
Diethyl
ether (ether) ε = 4.3 |
|
Hexane ε = 1.9 |