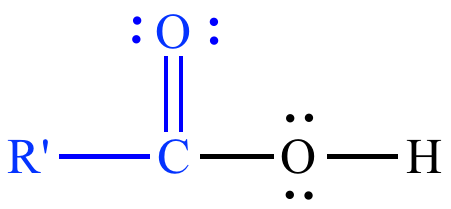
 |
+ |
 |
||
| Carboxylic
acid |
Alcohol |
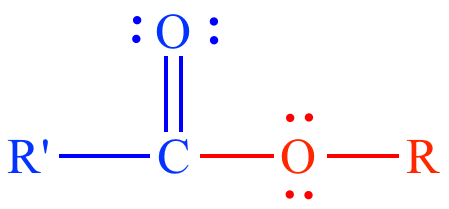
General carboxylate ester structure. |
 |
 |
 |
||
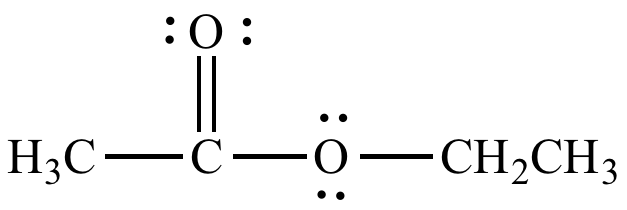
| Ethyl acetate, a common solvent. | |
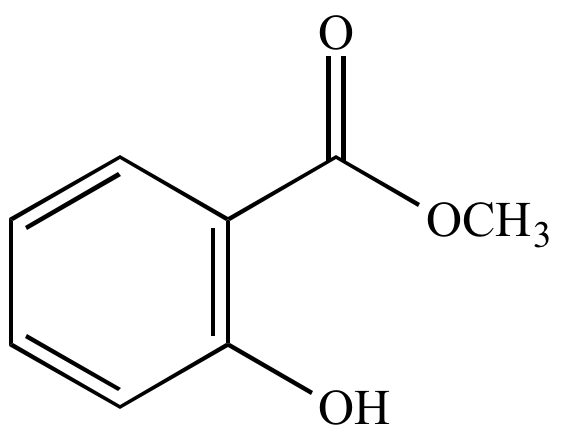
Methyl salicylate (oil of wintergreen). | |
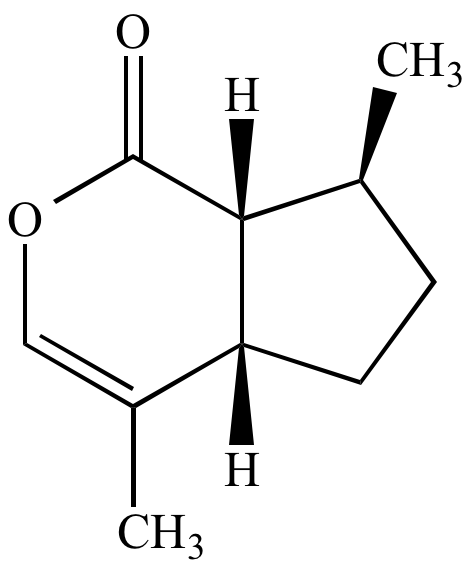
Nepetalactone, the active substance in catnip. |
| + |
 |
 |
||
| Methanol |
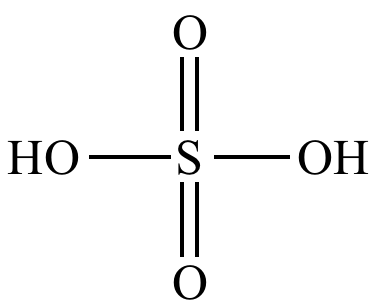
Sulfuric
acid |
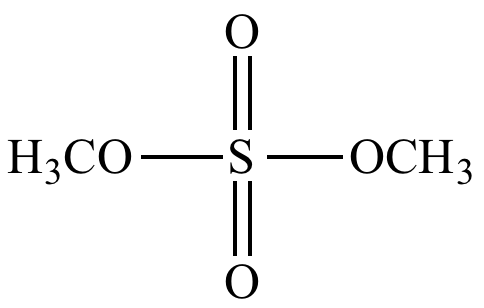
Dimethyl
sulfate,
a sulfate
ester. |
 |
+ |
 |
 |
|
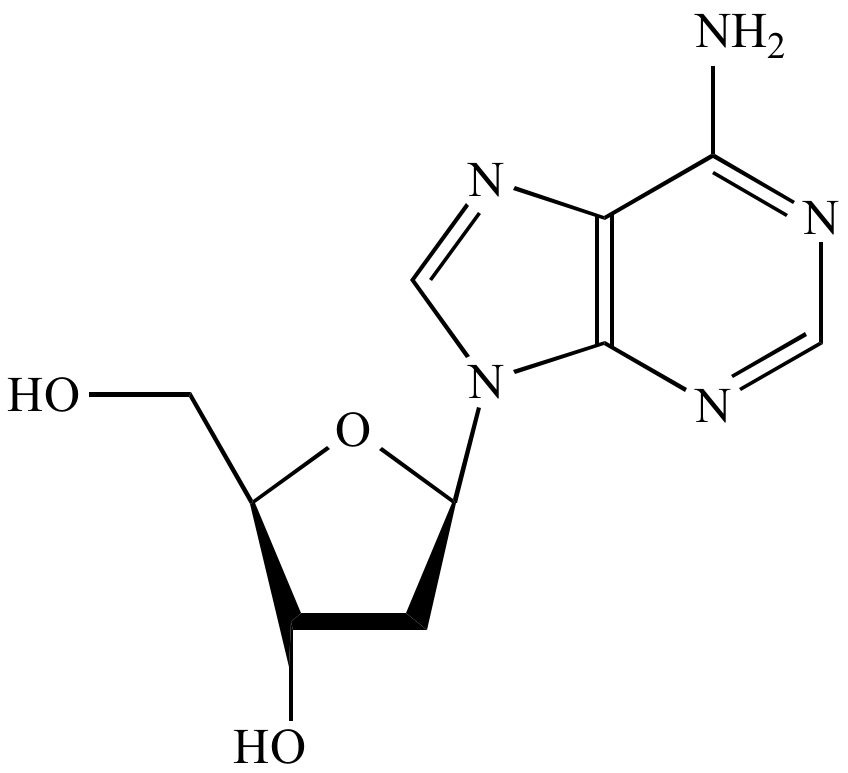
| A nucleoside |
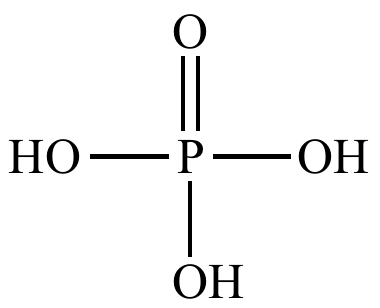
Phosphoric
acid |
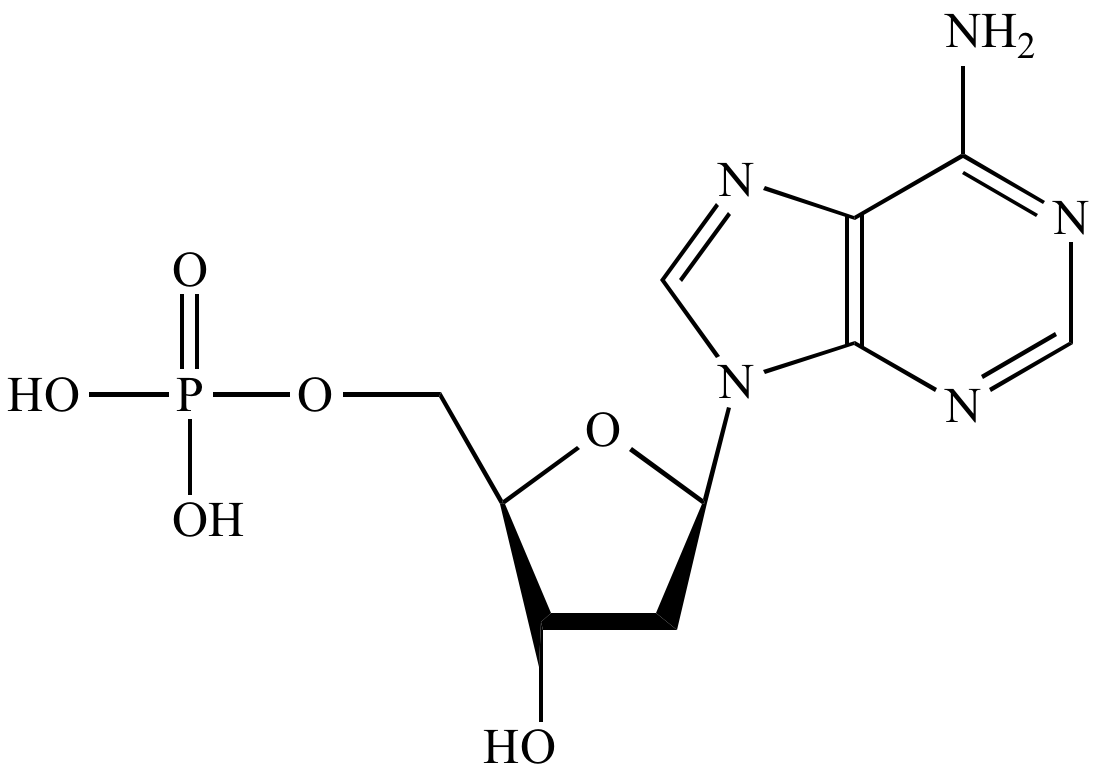
A nucleotide |