| |
 |
|
 |
 |
 |
 |
 |
|||||
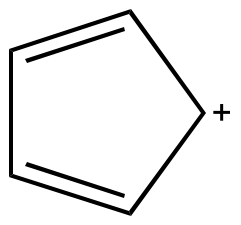
| Cyclopropenyl
cation Two pi electrons n = 0 |
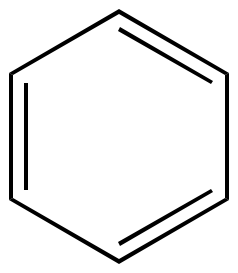
Benzene Six pi electrons n = 1 |
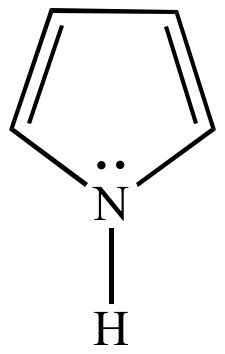
Pyrrole Six pi electrons n = 1 |
|
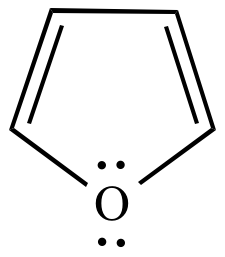
Furan Six pi electrons n = 1 |
|
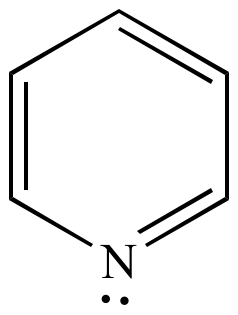
Pyridine Six pi electrons n = 1 |
|
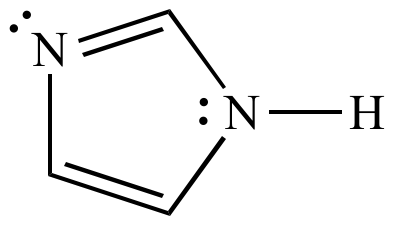
Imidazole Six pi electrons n = 1 |
|
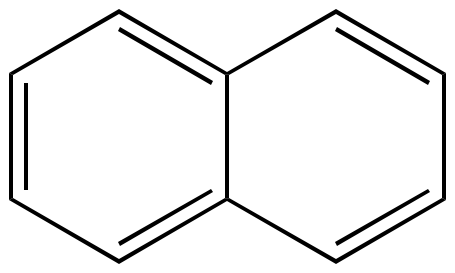
Naphthalene Ten pi electrons n = 2 |