 |
 |
|
| Hydrofluoric acid
dissolves glass, so it must be stored a container made of plastic (such as Teflon). |
|
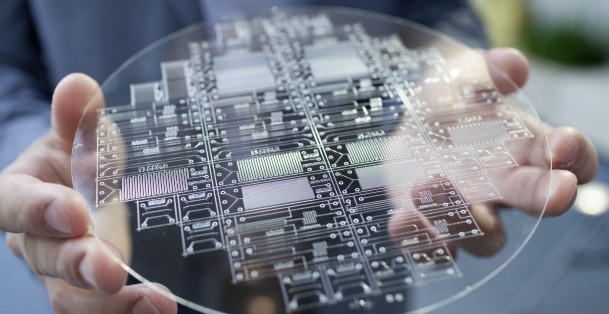
Glass that has been etched by hydrofluoric acid. |
 |
 |
|
| Hydrofluoric acid
dissolves glass, so it must be stored a container made of plastic (such as Teflon). |
|
Glass that has been etched by hydrofluoric acid. |