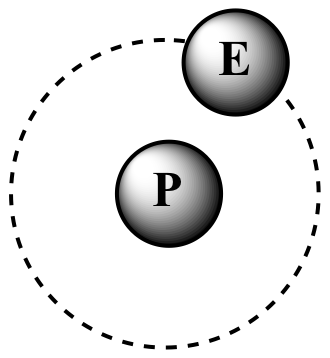
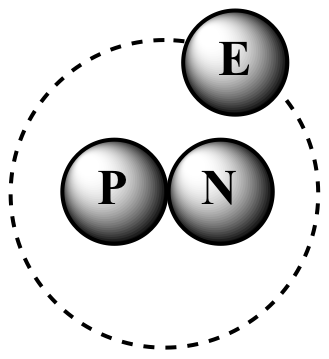
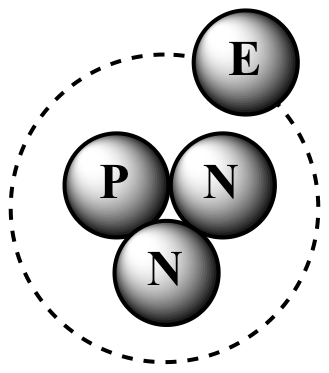
P = proton, N = neutron, E = electron
 |
 |
 |
||
| 1H One proton No neutrons |
|
2H
(deuterium) One proton One neutron |
|
3H
(tritium) One proton Two neutrons |