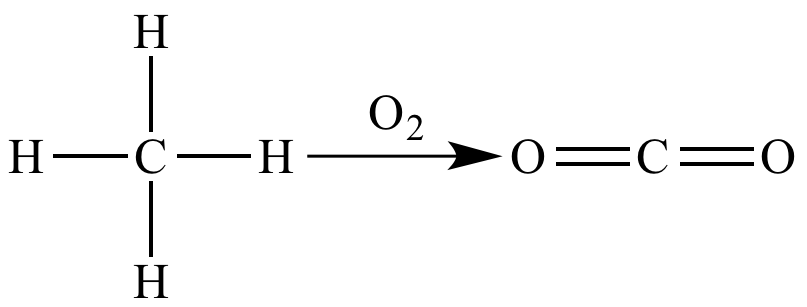
Combustion of methane is an oxidation reaction because the carbon atom is oxidized.
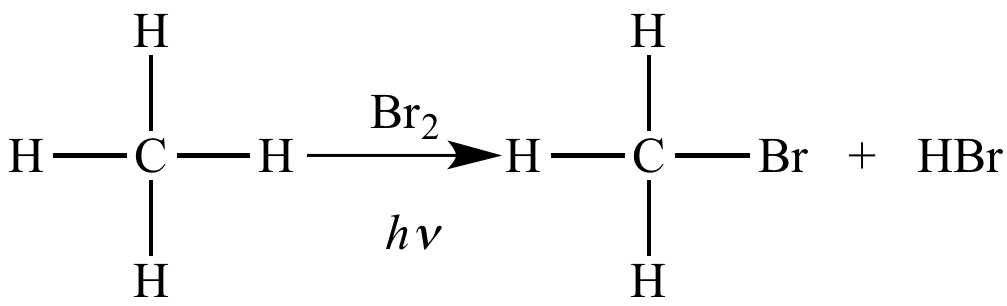
Free radical halogenation of methane is an oxidation reaction because the carbon atom is oxidized.
Rusting of iron is an oxidation reaction because Fe0 is oxidized to Fe3+.
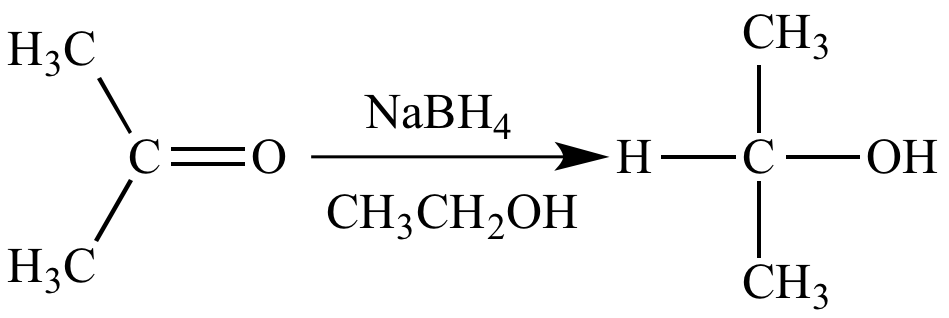
Reaction of sodium borohydride with acetone is a reduction reaction because the carbon atom of the carbonyl group is reduced.
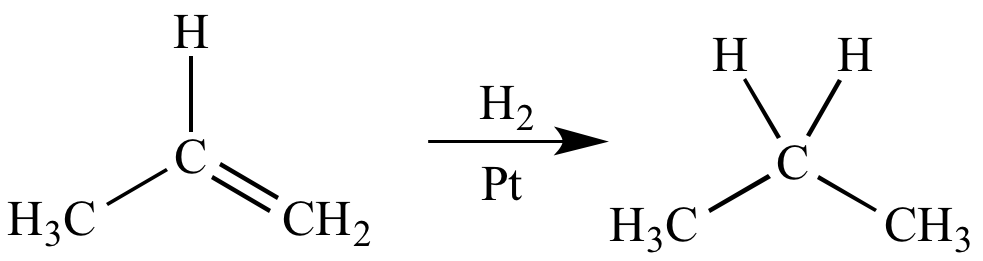
Catalytic hydrogenation of propene is a reduction reaction because the carbon atoms of the alkene are reduced.