
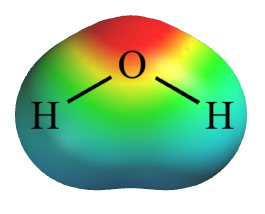
Red = higher negative charge.
Green = neutral.
Blue = higher positive charge.
 |
 |
|
| Hydrogen
bonding in water. |
|
Electrostatic
potential
map for water. Red = higher negative charge. Green = neutral. Blue = higher positive charge. |
 |
 |
|
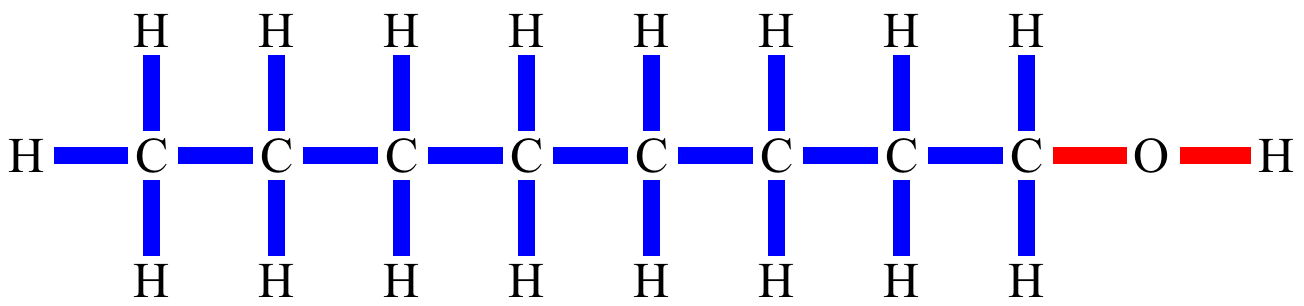
| 1-Octanol Nonpolar bonds in blue, polar bonds in red. |
|
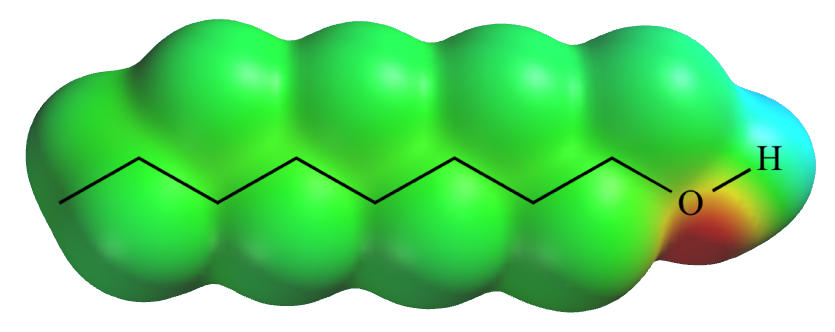
Electrostatic
potential
map for 1-octanol. Red = higher negative charge. Green = neutral. Blue = higher positive charge. |