 |
 |
|
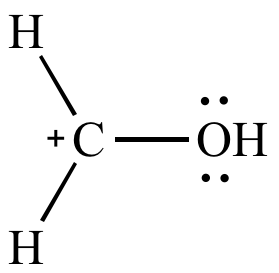
| Open
octet on carbon Less significant resonance contributor |
|
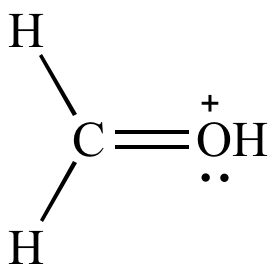
All
atoms have full
octets More significant resonance contributor |
Rule 2: The most significant resonance contributor has the least number of atoms with formal charges.
 |
 |
|
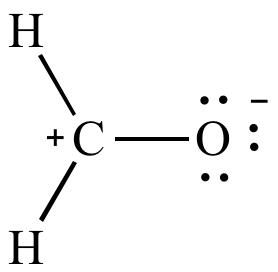
| Two
formal
charges Less significant resonance contributor |
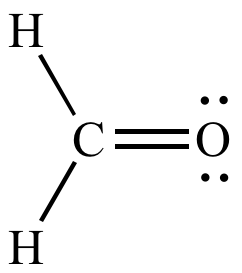
|
No formal
charges More significant resonance contributor |
Rule 3: If formal charges cannot be avoided, the most significant resonance contributor has the negative formal charges on the most electronegative atoms, and the positive formal charges on the least electronegative atoms.
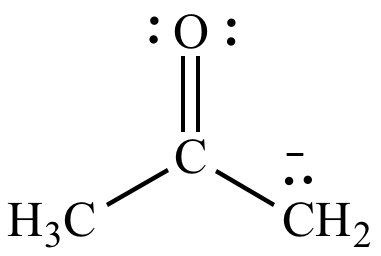 |
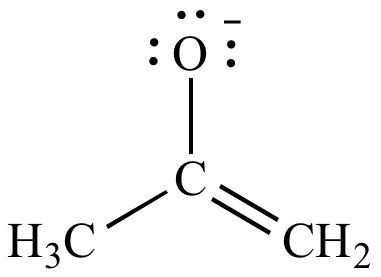 |
|
| Negative
formal
charge on carbon (EN
= 2.5) Less significant resonance contributor |
|
Negative
formal
charge on oxygen (EN
= 3.5) More significant resonance contributor |
Rule 4: The most significant resonance contributor has the greatest number of covalent bonds.
 |
 |
|
| Three
covalent
bonds Less significant resonance contributor |
|
Four
covalent
bonds More significant resonance contributor |
Rule 5: If a pi bond is present, the most significant resonance contributor has this pi bond between atoms of the same row of the periodic table (usually carbon pi bonded to boron, carbon, nitrogen, oxygen, or fluorine).
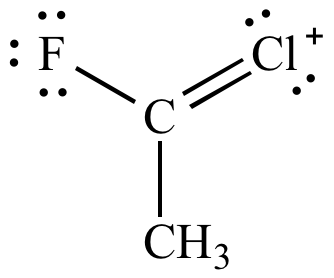 |
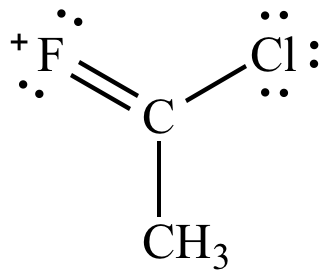 |
|
| Carbon-chlorine
double
bond Less significant resonance contributor |
|
Carbon-fluorine
double
bond More significant resonance contributor |
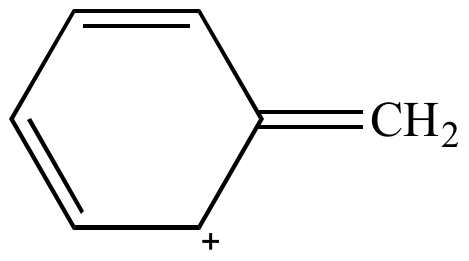
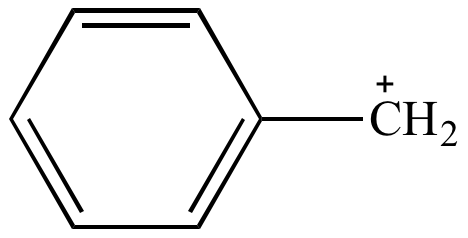
Rule 6: Aromatic resonance contributors are more significant than resonance contributors that are not aromatic.
 |
 |
|
| Nonaromatic Less significant resonance contributor |
|
Aromatic More significant resonance contributor |