| Liquid phase (H2O) |
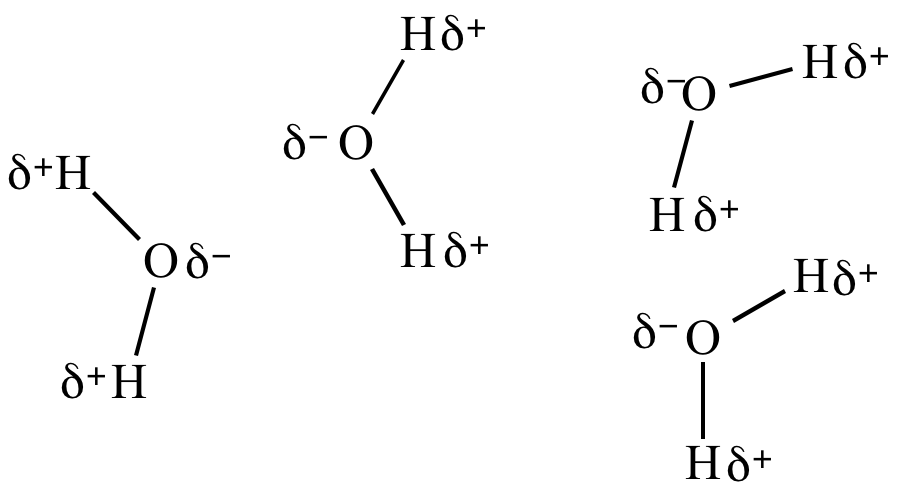 |
| Solid phase (NaCl) |
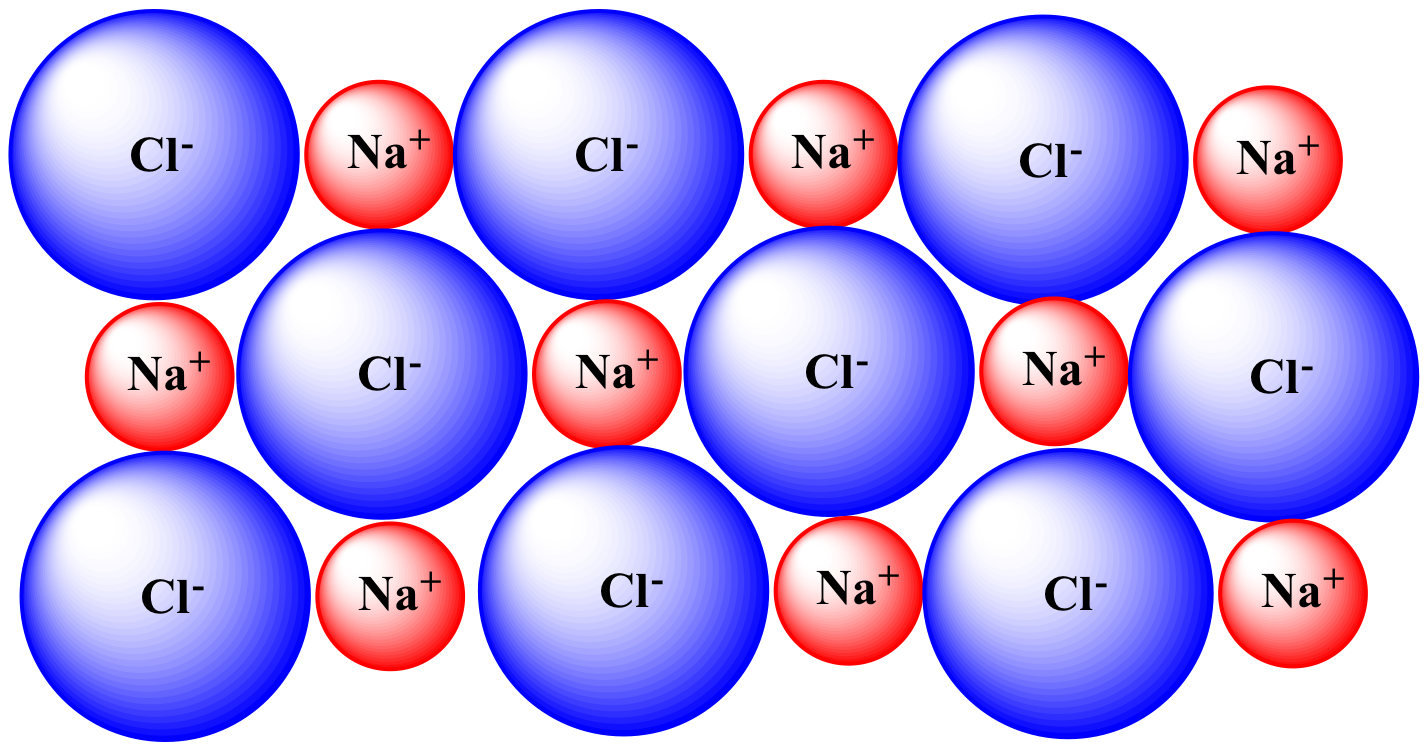 |
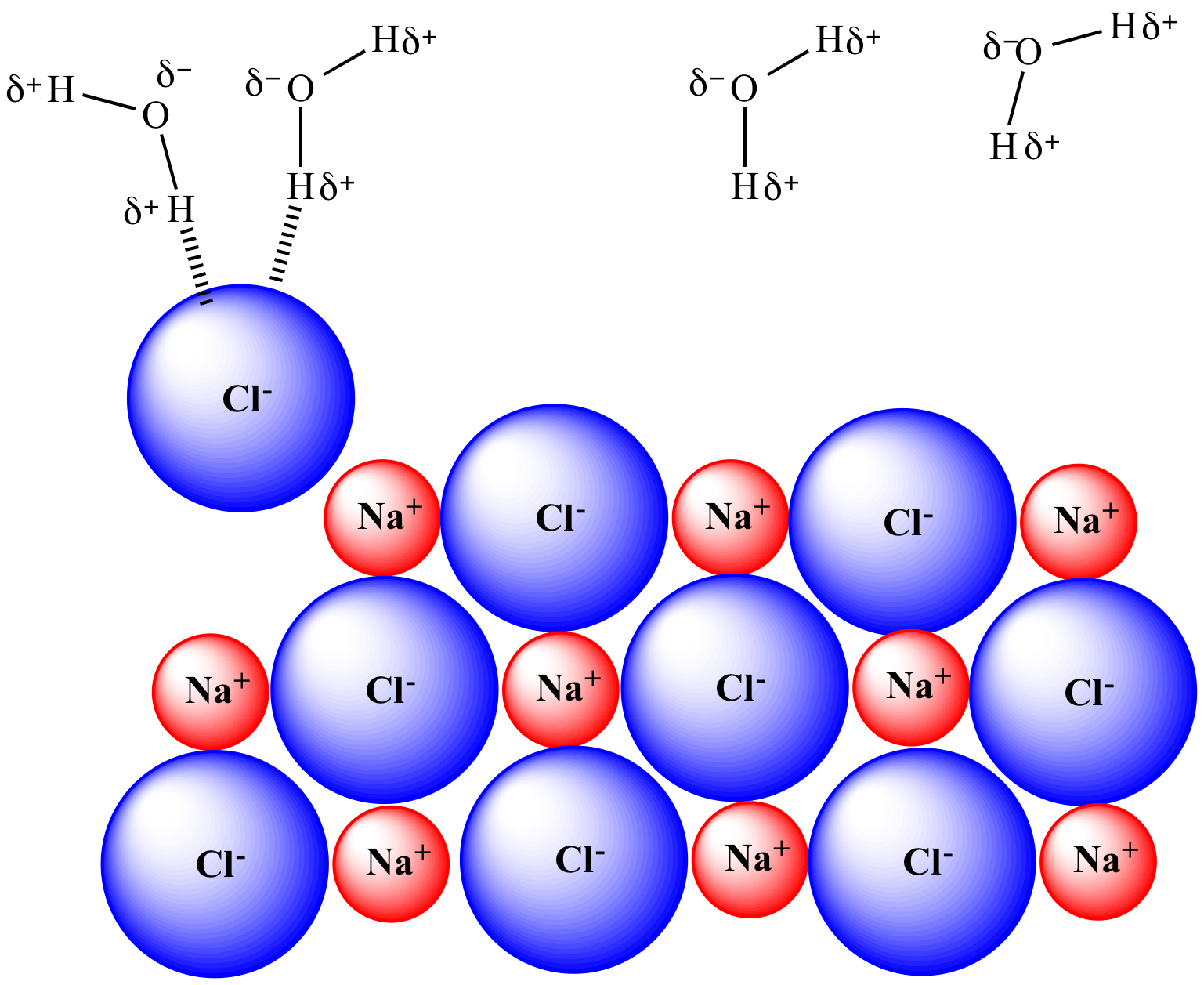
Partial solvation of one Cl- has occurred.
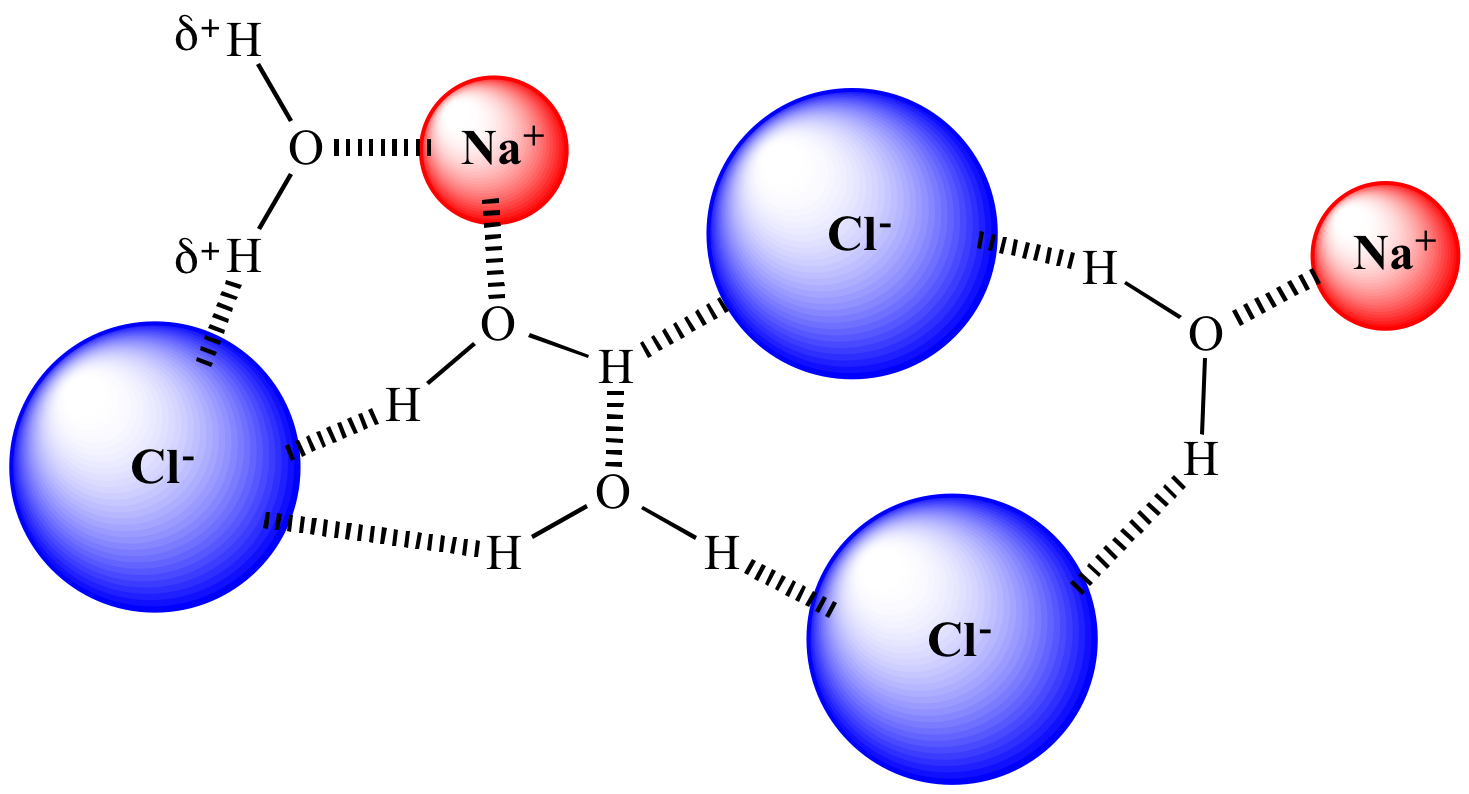
Complete solvation.
|
 |
 |
||||||
| NaCl completely undissolved. | NaCl begins to dissolve. Partial solvation of one Cl- has occurred. |
NaCl completely dissolved. Complete solvation. |