Torsional strain
(Pitzer strain):
Strain
caused by the close approach of atoms or groups
separated by three covalent
bonds. In the molecule
W-X-Y-Z, atoms W and Z may experience torsional strain
if a particular conformation
(such as an eclipsed
conformation) brings these atoms into close proximity.
Torsional strain
can cause a resistance to bond
rotation, and can influence a barrier
to
rotation. (Student confusion over the definitions of
torsional strain
versus other types of strain
was the impetus to start the Illustrated Glossary of Organic
Chemistry.)
| Sawhorse
projections: |
 |
 |
|
| Newman
projections: |
 |
 |
|
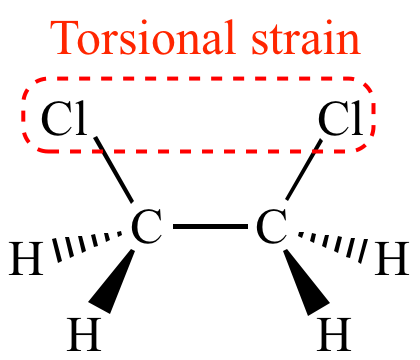
| Eclipsed
conformation More strain |
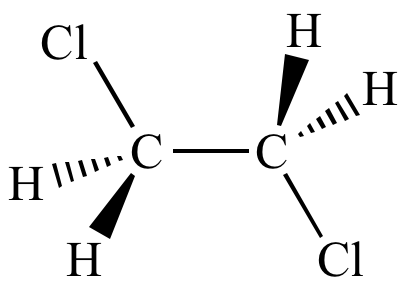
Anti-staggered
conformation Less strain |
When the chlorine atoms of 1,2-dichloroethane
are aligned (an eclipsed
conformation), the chlorine atoms experience torsional strain.
The eclipsed
hydrogen atoms also experience torsional strain
(albeit less than the chlorine atoms because hydrogen has a
smaller atomic
radius than chlorine). This torsional strain
is relieved when carbon-carbon bond
rotation changes the molecule
into a staggered
conformation (such as the anti-staggered
conformation shown here). Verify this with molecular
models.