Chemistry is a science of deduction. We'll never see or touch the fruits of of our synthetic labors and can only know that they exist and what their structure is by indirect evidence. Perhaps the greatest advantage of computer simulation to the working chemist is the ability to translate esoteric molecular information into readily understandable visual form. To let the sculptor see his creation.
The following are some of the graphics I've created at various times to illustrate chemical processes and properties. I believe these creations are as much art as science so I've brought them together for my own "Molecular Art Gallery." Most of these images were created in whole or in part with the Persistence of Vision ray tracing engine, Cambridge Scientifics Chem3D (for organizing the chemical structure information), my own proprietary software, and more than a little use of Adobe Photo Shop. As time permits, I'll add to this collection, and add links to the higher resolution versions of the ones in place. I hope you enjoy them.
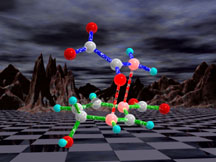
This graphic illustrates a "gating" mechanism by which incarcerated
acetonitrile molecules can exit a container molecule. Molecular gating
has been proposed as a method of site selective delivery of drugs
in biological systems. This graphic appeared on page 4 of the August
1996 issue of Chemical and Engineering News. These models also
appeared in the American Chemical Societies 1997 calendar.
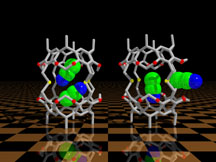
Houk group has modeled many different kinds of incarcerating molecules.
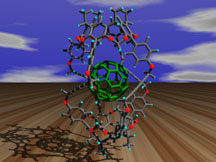
This graphic illustrates one of the more challenging systems modeled by
the Houk group--a proposed carcerand for selective trapping of "Bucky
Balls." A potential method for purifying Buckminster Fullerene, this
model was chosen as a
"Image of the Week" at the San Diego
Supercomputer Facility.