 Ionic Equilibria
Ionic Equilibria Solubility of Salts
Solubility of SaltsLearn Table 7.1 on page 253.
 Ionic Equilibria
Ionic Equilibria
Since solids don't go into the mass action equation we can define an equilibrium solubility product as,
Some notes on Ksp:
1) Ksp expressions are not written for highly soluable salts because such solutions are too nonideal. Use Ksp only for slightly soluable salts.
2) Ksp values come from experiment and widely vary. Numerical values are usually good to only two significant figures.
3) Ksp depend on temperature. Usually it increases as temperature increases.
 Precipitation and Ksp
Precipitation and Ksp
 Complex Ions and Solubility
Complex Ions and Solubility
Since all species are aqueous they enter into the formation constant,
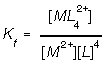
Metal ions gain ligands one by one. In the above example the metal gains its ligand one after the other, which each gain represented by a K. We can approximate this if the ligan is present in excess and the K's are fairly large, then
1) Most of the metal ion will be tied up in the complex with the most ligands.
2) The concentration of free ligands will approximately equal the concentration of excess ligand that is calculated assuming complete coordination.