The generation of force that drives muscle contraction and cell motility involves translation of chemical energy from ATP into mechanical work via interactions between actin and mysoin. The actomyosin system (Figure 1) has been studied extensively as a model of chemomechanical transduction, with a major focus on the conformational changes arising within actin and myosin that lead to force generation.

My work focuses on: (1) Determining residues in actin that are important in the actomyosin interaction; and, (2) Testing the hypothesis that specific charged residues in the N-terminus of actin are important in the transition from weakly- to strongly-bound actomyosin complexes.
The chemomechanical transduction of ATP energy to force and motion occurs through a series of specific interactions between myosin and actin. The mechanism of force generation currently accepted as standard is the "Rotating Crossbridge Cycle." (Figure 2)
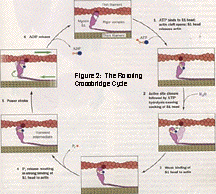
Beginning with a strongly-bound, nucleotide-free actomyosin complex (rigor), ATP-binding and subsequent hydrolysis yields a weakly-bound actomyosin state (A-M-ADP-Pi). Analogously, initial ATP-binding immediately dissociated the actomyosin complex with ATP hydrolysis occurring on the detached myosin; the weakly-bound complex results upon rebinding of myosin-ADP-Pi to actin. The weakly-bound A-M-ADP-Pi complex undergoes a conformational change to a strongly-bound, force-bearing, A-M*-ADP-Pi crossbridge. The "powerstroke" of muscle contraction is thought to occur within the subsequent steps: phosphate release (which results in another strongly-bound, force-bearing state, A-M*-ADP) and ADP release (which returns the crossbridge to the beginning of the cycle). Since the transition from weakly- to strongly-bound actomyosin states directly precedes the powerstroke, free energy resulting from this transition (A-M-ADP-Pi to A-M*-ADP-Pi) is considered to be instrumental in the transduction of the chemical energy of ATP to mechanical force.
My work focuses on characterization of the molecular mechanisms that occur during the force-generating weak-to-strong transition. The conformational changes that lead to force generation in this transition must occur within an actomyosin crossbridge. The specific contacts and interactions within the interface between actin and myosin are thus important.
The rigor-complexed actomyosin model suggests contacts between the 50kDa region of S1 and subdomains 1 and 2 of an actin monomer within F-actin. Specifically, negatively-charged surface residues (aa 1-4, D24/D25, and E99/E100) on actin are modeled in close proximity to positively-charged lysines within a flexible surface loop at the 50/20 kDa junction of S1.
Using yeast mutant actins in which the N-terminal charged residues have been neutralized (Charge-to-Alanine mutants), I'll be able to determine the result upon loss of this electrostatic interaction between actin and myosin.
 Back to
Wenise's Homepage
Back to
Wenise's Homepage