| |
The
design and construction of chemical structures is at the heart of what chemist
do, however, it remains more of an art than a science. This is largely due to
the difficulty in predicting the outcome of chemical synthesis in particular
those of large molecules and extended structures. To accomplish the synthesis
of structures by design, it is important (1) to control the orientation
(connectivity and geometry) of the building blocks in a targeted structure, and
(2) to obtain the products in crystalline form so that their atomic arrangement
can be definitively characterized by X-ray diffraction techniques. Indeed, the
challenges are conceptual and practical in that one needs to know what
structures might form from a given set of molecular building blocks and then
find means of linking such building blocks into specific predetermined
structures.
My
research group has been developing the conceptual as well as the practical
aspects of constructing chemical structures using the concept of molecular building
blocks. Our research has led to the invention of new classes of crystalline
porous materials most notably ones that we call metal-organic frameworks
(MOFs). In general, MOF structures have two main components: the organic
linkers and the metal oxide units. The linkers act as ‘struts’ that bridge the
metal oxide units which in turn act as ‘joints’ in the resulting MOF
architecture. This arrangement inevitability produces porous structures for
which we’ve shown that molecules can pass through the pores with preservation
of the MOF structure. Since we reported the first porous MOFs in 1998 and 1999,
over 2,000 three-dimensional structures have been reported by my group and
others around the world. It is worth noting that to date there are more porous
MOFs than porous zeolites or carbon materials, and MOFs have extraordinary
surface areas (2,000-6,500) exceeding those of state-of-art materials.
Furthermore, MOFs are stable in air at room temperature and withstand
temperatures up to 450°C. They are made using simple, inexpensive and high
yielding solution synthesis methods.
These
properties coupled with the flexibility with which one can vary the composition
and metrics of the metal oxide units and the organic links of a given
structure, have led to extensive investigation of MOFs in both academia and
industry. At the heart of the matter is that we’ve shown for the first time
that chemists are able to predictably assemble molecular building blocks into
predetermined structures which can be functionalized and their metrics altered
at will. We call this new kind of chemistry ‘reticular chemistry’ to emphasize
that the dream of designing large and extended structure is becoming a reality.
We define reticular chemistry as the chemistry dealing with linking of molecular
building blocks, by strong bonds, into predetermined structures.
It
is worth discussing aspects of reticular chemistry here to point out the
thinking involved in the design of new structures. In recent contributions we
reported how the concept of secondary building units (SBUs) is being applied
with eminent success to the design of highly porous and rigid MOF structures.
Here, clusters such as those of the copper-carboxylate paddlewheel, Cu2(O2C-)4,
and the octahedral basic zinc carboxylate, Zn4O(O2C-)6,
have been used as rigid SBUs that respectively act as square and octahedral
joints (i.e. vertices) in the framework.
If the designer identifies the one step reaction conditions that reproducibly
lead to such a particular SBU, then control of the vertex geometry in the
resulting MOF is possible. Since the organic links remain intact and their
geometry preserved throughout the assembly process, one should also be able to
predict the underlying topology of the resulting MOF structure.
However,
the number of topologies that could, in principle, result from linking
molecular shapes into extended MOF structures is vast—giving rise to
three questions which my group is actively engaged in addressing: (1) From this
large topology space, how do we as designers identify the most important
topologies that should be considered in this chemistry? (2) How are they
distributed among crystal structures already reported in the chemical
literature? (3) How can these data be interpreted, organized, and classified
for the purpose of developing systems of ‘grammar’ and ‘taxonomy’ that can lead
to the design and construction of extended structures, the rationalization of
existing structures, and the prediction of new ones?
Our
ability to design and synthesize a MOF structure nearly at will is being used
to provide solutions to problems of energy storage (hydrogen and methane),
carbon dioxide sequestration, separation of gases for medical uses,
polymerization catalysis, highly selective and sensitive sensors, and countless
others currently under investigation by my group and by chemical, automobile
and electronic industries. We work closely with companies; an aspect that
provides my group members (undergraduates and doctorate graduate students,
postdoctoral fellows, and research fellows) with a unique experience of being
engaged in a spectrum of projects involving basic science of design and
synthesis, characterization of structure and porosity, and the feasibility of
MOFs in various applications.
The
excitement of reticular chemistry and the ability to construct chemical
structures from molecular building blocks has recently led us to create new
classes of materials potentially as extensive as MOFs: metal-organic polyhedra
(MOPs), zeolite imidazolate frameworks (ZIFs) and covalent organic frameworks
(COFs). On a fundamental level, these classes of materials are produced by
linking molecular building blocks through progressively stronger and stronger
bonds, and doing so and still overcoming the ‘crystallization problem’. What kind
of new chemistry and basic science concepts will these new materials inspire?
What kind of new properties will be uncovered? What kind of new applications
will be developed? And what immense pleasure will be derived in addressing
these questions…
A partial list of ongoing projects in the Yaghi research
group is provided here.
- Design and Synthesis,
structure characterization and porosity of MOFs, MOPs, ZIFs, and COFs
- Automated and High throughput
methods for inorganic synthesis and for X-ray powder diffraction studies
of porous crystals
- Design and synthesis of
electronically conducting porous frameworks for highly selective and
sensitive sensing
- Gas storage and transport:
Hydrogen storage for automobile fueling and mobile electronics; methane
storage for automobile fueling and for transport of natural gas reserves;
carbon dioxide separation and storage applications: power plants and
automobile emissions; fuel cell applications including the separation of
CO
- Polymerization catalysis by
MOFs for the production of polymers used as construction materials
- Biomedical applications
including drug molecule storage separation, storage and release by MOFs
Learn
more about Prof. Yaghi in recent interviews published on the web:
http://www.umich.edu/news/index.html?Releases/2005/Nov05/yaghi
http://www.sciencewatch.com/nov-dec2004/sw_nov-dec2004_page3.htm
Metal-Organic
Frameworks
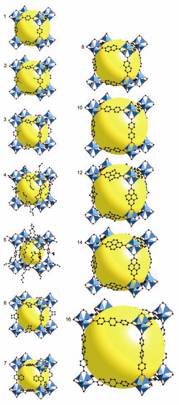
A
Covalent Organic Framework
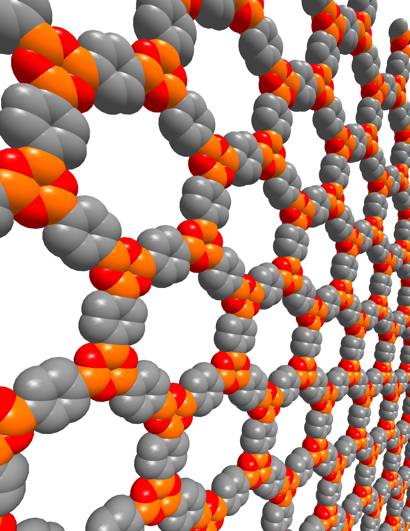
- Robert
W. Parry Collegiate Chair, University of Michigan
- Sacconi
Medal, Italian Chemical Society, Division of
Inorganic Chemistry
- Ranked
among the top most-highly cited chemists (over
100 citations per paper)
- Department
of Chemistry Chair's Excellence in Research
Award, University of Michigan
- 3M
Faculty Award
- Graduate
College Mentor Award
- Exxon
Award, American Chemical Society-Solid State
Chem. Division
- Polymer-induced heteronucleation for the
discovery of new extended solids, A. Grzesiak, F. Uribe, N. Ockwig, O. M.
Yaghi, A. Matzger, Angew. Chem. Int. Ed., 2006, 118, 2615-2618.
- A metal-organic framework with a
hierarchical system of pores and tetrahedral building blocks, A. Sudik, A.
Cote, A. Wong-Foy, M. O'Keeffe, O. M. Yaghi. Angew. Chem. Int. Ed.,
2006, 118, 2590-2595.
- A microporous metal-organic framework for
gas-chomatographic separation of alkanes, B. Chen, C. Liang, J. Yang, O.
M. Yaghi. Angew. Chem. Int. Ed., 2006, 118,
1390-1393.
- Determination of the hydrogen absorption
sites in Zn4O(1,4-benzenedicarboxylate) by single crystal
neutron diffraction, E. Spencer, J. Howard, G. McIntyre, O. M. Yaghi Chem.
Comm., 2006 (3), 278-280.
- Effects of functionalization, catenation,
and variation of the metal oxide and organic linking units on the
low-pressure hydrogen adsorption properties of metal-organic frameworks, J.
Roswell, O. M. Yaghi. J. Am. Chem. Soc., 2006, 128,
1304-1315.
- Metal-organic frameworks with exceptionally
high capacity for storage of carbon dioxide at room temperature, A.
Millward, O. M. Yaghi. J. Am. Chem. Soc., 2006, 127,
17998-17999.
- Characterization of H-2 binding sites in
prototypical metal-organic frameworks by inelastic neutron scattering, J.
Rowsell, J. Eckert, O. M. Yaghi. J. Am. Chem. Soc., 2006, 127,
14904-14910.
- What do we know about three-periodic nets?
O. Delgado-Friedrichs, M. D. Foster, M. O'Keeffe, D. M. Prosperio, M. Treacy,
O. M. Yaghi, J. Solid State Chem., 2005, 178,
2533-2554.
- Reticular chemistry -
Present and future prospects - Introduction, M. O'Keeffe, O. M.
Yaghi
J. Solid State Chem., 2005, 178, V-VI.
- Porous, crystalline,
covalent organic frameworks, A. P. Cote, A. Benin, N. Ockwig,
A. Matzger, M. O'Keeffe, O. M. Yaghi. Science, 2005, 310,
1166.
- Porous Metal-Organic Truncated Octahedron
Constructed from Paddle-Wheel Squares and Terthiophene Links, Z. Ni, A. Yasser,
T. Antoun, O. M. Yaghi J. Am. Chem. Soc., 2005, 127,
12752.
- Raman Spectra of Hydrogen and Deuterium
Adsorbed on a Metal-Organic Framework, A. Centrone, D. Y. Siberio-Pérez,
A. R. Millward, O. M. Yaghi, A. J. Matzger, G. Zerbi, Chem. Phys. Lett.,
2005, 411, 516.
- Gas
Adsorption Sites in a Large-Pore Metal-Organic Framework, J.Rowsell, E.
Spenser, J. Eckert, J.A. K. Howard, O. M. Yaghi. Science, 2005,
309, 1350.
- Design,
Synthesis, Structure, and Gas (N2, Ar, CO2, CH4
and H2) Sorption Properties of Porous Metal-Organic Tetrahedral
and Heterocuboidal Polyhedra, A. Sudik, N. Ockwig, A. Millward, A.
Cote, O. M. Yaghi, J. Am. Chem. Soc.. 2005, 127, 7110.
- Metal-Organic
Frameworks Based on Trigonal Prismatic Building Blocks and the New 'acs'
Topology,
A. Sudik, N. Ockwig, A. Cote, O. M. Yaghi, Inorg. Chem. 2005,
44, 2998.
- High
H2 Adsorption in a Microporous Metal-Organic Framework with
Open-Metal Sites, B. Chen, D. S. Contreras, N. Ockwig, O. M. Yaghi, Angew.Chem.
Int. Ed. 2005, 44, 4745. (Featured on cover)
- Strategies
for Hydrogen Storage in Metal-Organic Frameworks, J. Rowsell, O. M. Yaghi,
Angew. Chem. Int. Ed.
2005, 44, 4670. (Featured on cover)
- Reticular
Chemistry: Occurrence and Taxonomy of Nets, and Grammar for the Design of
Frameworks, N. Ockwig, O. D. Friedrichs, M. O'Keeffe, O. M. Yaghi, Acc.
Chem. Res. 2005, 38, 176.
- Transformation
of a Metal-Organic Framework from the NbO to PtS Net, B. Chen, N. Ockwig, F. R. Fronczek, D.
S. Contreras,
O. M. Yaghi, Inorg. Chem., 2005, 44, 181.
- Rod-Packings
and Metal-Organic Frameworks Constructed from Rod-Shaped Secondary
Building Units, N. Rosi, J. Kim, B. Chen, M. Eddaoudi, M. O'Keeffe, O. M.
Yaghi. J. Am. Chem. Soc., 2005, 127, 1504.
- Metal-Organic
Frameworks: A New Class of Porous Materials, J. Rowsell, O. M. Yaghi,
Micro- and Mesoporous Mater., 2004, 73, 3.
- Structural
Study of New Hydrocarbon Nano-Crystals by Energy-Filtered Electron
Diffraction, J. Wu, N. Melcer, W. Sharp, M. O'Keeffe, JCH Spence, O. M.
Yaghi, Ultramicroscopy, 2004, 98, p. 145.
- Hydrogen Sorption in
Functionalized Metal-Organic Frameworks, J. Rowsell, A.
Millward, K. Park, O. M. Yaghi, J. Am. Chem.
Soc. 2004, 126,
p. 5666.
- Design
of New Materials for Methane Storage, T. Duren, L. Sarkisov, O. M. Yaghi,
R. Q. Snurr, Langmuir, 2004, 20, 2683.
- A
Route to High Surface Area, Porosity and Inclusion of Large Molecules in
Crystals,
H. Chae, D. Y. Siberio-Perez, J. Kim, Y. Go, M. Eddaoudi, A. Matzger, M.
O'Keeffe, O. M. Yaghi, Nature, 2004, 427, p. 523. (Featured
in (1) Chemical & Engineering News magazine, Feb. 9, 2004, (2) BBC
World Service, Feb. 04, (3) New Scientist, Feb. 04, and (4) several
science magazines and localpapers)
- Three-Periodic
Nets and Tilings: Minimal Nets. C. Bonneau, O. D. Friedrichs, M. O'Keeffe, O.
M. Yaghi, Acta Cryst., 2004, A60: p. 517.
- Three-Periodic
Nets and Tilings: Regular and Quasiregular Nets, O. D. Friedrichs,
M. O'Keeffe, O. M. Yaghi, Acta Cryst., 2003, A59:
p. 22.
- Three-Periodic Nets and Tilings:
Semiregular Nets, O. D. Friedrichs, M. O. O'Keeffe, O. M. Yaghi, Acta Cryst.,
2003, A59: p. 515.
- Reticular Synthesis and the Design of New Materials, O.
M. Yaghi, M. O'Keeffe, N. Ockwig, H. K. Chae, M. Eddaoudi, J. Kim, Nature, 2003, 423,
p.705.
- Metal-Organic
Frameworks as New Materials for Hydrogen Storage, N. Rosi, M.
Eddaoudi, D. Vodak, J. Eckert, M. O'Keeffe, O. M. Yaghi, Science, 2003,
300, p. 1127. (Featured in (1) Chemical & Engineering News
magazine, May 19, 2004, and (2) Technology Research News Magazine, May 21,
03)
- Computation of Aromatic C3N4 Networks
and Synthesis of the Molecular Precursor N(C3N3)3Cl6, D.
T. Vodak, K. Kim, L. Iordanidis, P. Rasmussen, M. O'Keeffe, A. Matzger, O. M.
Yaghi, Chem. Eur. J., 2003, 9,
p. 4197.
- The
CdSO4, Rutile, Cooperate and Quartz Dual Nets: Interpenetration
and Catenation,
O. D. Friedrichs, M. O'Keeffe, O. M. Yaghi. Solid State Sciences, 2003,
5, p. 73.
- Design
of Frameworks with Mixed Triangular and Octahedral Building Blocks
Exemplified by the Structure of [Zn4O(TCA)2] Having
the Pyrite Topology,
H. K. Chae, J. Kim, O. Delgado Friedrichs, M. O'Keeffe, O. M. Yaghi, Angew.
Chem. Int. Ed., 2003, 42, p. 1819.
- Cd16In64S13444:
35 Å Tetrahedron with a large Cavity, H. Li, J. Kim, O. M. Yaghi,
Angew. Chem. Int. Ed., 2003, 42, p.
1819. (Featured on cover)
- Synthesis and Characterization of Zirconogermanates,
J. Plevert, R. S.-Smith, T. Gentz, H. Li, T. L. Groy, M. O'Keeffe, O. M. Yaghi,
Inorg. Chem. 2003, 42, p.
5954.
- Layered Structures Constructed from New
Linkages of Ge7(O,OH,F)19 Clusters, J. Plevert,
T. Gentz, T. L. Groy, M. O'Keeffe, O. M. Yaghi, Chem. Mater.
2003, 15, p.
714.
Complete List of Publications is availabe on the
Yaghi Group Web Site.
Department of Chemistry & Biochemistry
UCLA
Box 951569 (post)
607 Charles E. Young Drive East (courier)
Los Angeles, CA 90095-1569
Yaghi Group Web Site
|


