last updated
Drying Agents
Drying agents (also called desiccants) come in various forms and have found widespread use in the foods, pharmaceuticals, packing, electronics and many manufacturing industries. A desiccant is a hygroscopic substance that induces or sustains a state of dryness in its vicinity. Ideally, it is chemically stable and chemically inert (i.e., silica). Unfortunately, this is not always the case in the chemistry lab because the drying agent comes into direct contact with the solvent and the chemical.

Many organic solvents are immiscible with aqueous solutions, but they are able to dissolve significant amounts of water because of their polarity i.e., diethyl ether dissolves 7 % of its weight in water while tetrahydrofuran is completely miscible with water (Why?). Unfortunately, water is a compound that is very difficult to remove from many compounds, because they are either holding on to it well (i.e., alcohols) or the compound itself is steam volatile. Bottom-line is that the more polar the solvent is, the more hygroscopic it will usually be because it dissolves the water better. Thus, removing water and other impurities from a solution can become an arduous task but is necessary if the reagents are also sensitive towards water i.e., Grignard reagents or in cases where water has a detrimental effect on the yield or rate of the reaction. In those cases, drying agents like calcium hydride (CaH2), sodium metal (in combination with benzophenone) or lithium aluminum hydride (LiAlH4) are used to chemically destroy the water in the solvent. Those compounds are relatively reactive and difficult to handle and usually not used in lower division undergraduate laboratories (see below).
Use of Conventional Drying Agents
Commonly used drying agents in organic laboratories are calcium chloride (CaCl2), sodium sulfate (Na2SO4) calcium sulfate (CaSO4, also known as Drierite) and magnesium sulfate (MgSO4), all in their anhydrous form. How do they work? All four of them readily form hydrates at low temperatures according to
![]()
Their efficiency is measured by intensity, capacity and velocity can greatly vary from one solvent to the other. Capacity refers to the maximum numbers of moles of water that the drying agent can bind (n). Another parameter of importance is the efficiency, which refers to the amount of water left in the organic solution after the drying process is completed (e).
1. Calcium chloride (n=6, e=1.5 mg/L) is a very good drying agent for a broad variety of solvents but is generally not compatible with hydroxy (alcohol, phenol), amino (amine, amide) and carbonyl (acid, ketone, ester) functions due to basic impurities such as Ca(OH)2 and CaCl(OH). In addition, it tends to form adducts with
some of those compounds as well. It is often used in drying tubes
because it also is available in granular form.
2. Calcium sulfate (n=0.5, e=0.004 mg/L) is a neutral and good drying agent. However, it does not have a high capacity, which makes it useless for very wet solutions. The commercially available Drierite® contains
about 2 % cobalt chloride as indicator, which can be leached out into various solvents i.e. ethanol, DMSO, DMF, ethers, etc. Drierite is often used in desiccators. If the compound is pink, the water can be removed by heating the compound to 210 oC for an hour.
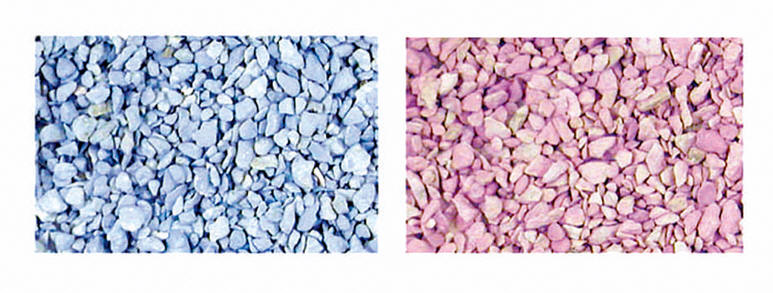
Dry (blue)
Wet (wet)
3. Magnesium sulfate (n=7, e=2.8 mg/L) is a slightly acidic drying agent. It works well in solvents like diethyl ether, but not as well for ethyl acetate.
It is a fast drying agent, in part because it comes as a fine powder with a large surface area.
4. Sodium sulfate (n=10, e=25 mg/L) has a very high capacity and is mainly used for very wet solutions. It is very efficient in ethereal solutions, but it also absorbs other polar compounds like alcohols, etc. In addition, it is slower compared to magnesium sulfate, etc.
5. Potassium hydroxide (KOH, n= high, e=0.1 mg/L) and potassium carbonate (K2CO3, n=2, e=moderate) are both of basic nature and often used to dry basic solutions containing amines. They cannot be used to dry acidic compounds since they react with them.
6. Sulfuric acid (H2SO4, e=0.003 mg/L) and phosphorous pentoxide (P4O10) are both acidic drying agents that are mainly used in desiccators and not in direct contact with the solution since they are very aggressive reagents. Both have a very high capacity. Sulfuric acid forms hydrates while phosphorous pentoxide is ultimately converted into phosphoric acid.
P4O10 + 6 H2O ----- > 4 H3PO4
7. Molecular sieves are alumino silicates with a three-dimensional network with different pore sizes (3-5 Å). They have to be activated prior use and also can be regenerated at higher temperatures (~180 oC - 260 oC, 1-2 hours).
| Class of Compounds | Recommended Drying Agent |
| Alkane, alkyl halides | MgSO4, CaCl2, CaSO4, H2SO4, P4O10 |
| Aromatic hydrocarbons, ethers | MgSO4, CaCl2, CaSO4, P4O10, Na-metal |
| Aldehydes, ketones, esters | Na2SO4, MgSO4, K2CO3, CaSO4 |
| Alcohols | MgSO4, K2CO3, CaSO4, CaO, BaO |
| Amines | KOH, K2CO3 |
| Acidic compounds | Na2SO4, MgSO4, CaSO4 |
One of the main problems in the drying process is that the equilibrium above is shifted to the left if the mixture is heated (increase in entropy!) in most cases unless the water is chemically destroyed. Therefore, the drying agent has to be removed (by filtration or decanting) from the dry solution prior to removal of the solvent. Drying agents like Drierite or molecular sieves can be recycled several times by heating them to an appropriate temperature (see above).
Example: Reversibility of drying process
The following data can be obtained from the literature:
| Compound | ΔH(kJ/mol) | ΔS(J/mol*K) |
| Na2SO4 |
-1387.1
|
149.6
|
| Na2SO4*10 H2O |
-4322.9
|
587.9
|
| H2O |
-285.8
|
69.95
|
| MgSO4 |
-1284.9
|
91.6
|
| MgSO4*7 H2O |
-3388.0
|
372.0
|
The Gibbs free energy for the following reaction
Na2SO4 + 10 H2O ---- > Na2SO4 * 10 H2O
can be described by
ΔG = ΔH - TDS = -77.8 kJ/mol - T (-261 J/mol*K)
Based on this equation, it can be estimated that the reaction reverses at 298 K or 25 oC (ΔG =0). The drying process itself is slightly exothermic (ΔH for the process is negative) but the entropy decreases during the drying process. The solution often times warms up a little bit when the drying agent is added.
Practical Aspects for the Use of Conventional Drying Agents
One of the main problems is that many drying agents do not only absorb water, but also other polar compounds. Hence, an excess of drying agent should be avoided in order to prevent the absorption of the target compound, particularly if the compound
was polar as well. Even though water usually has a higher affinity towards the drying agent, excess can also lead to significant loss of product. Note that the presence of other polar compounds i.e. alcohols, etc increase the solubility of water in the organic solvent.
How do I know that I added enough drying agent?
Before a drying agent is added, the organic layer has to be separated as thoroughly as possible from the aqueous layer. It does make very little sense to add the drying agent if there is a second layer on the bottom (or the top depending which organic solvent is used). The experimenter
has to make sure that the "organic layer" is really the organic layer.
The drying process does take some time to complete. Most students are not patient enough and add too much drying agent right from the start and often lose a significant amount of their product this way. The best protocol is to add a small amount first. The mixture is swirled and then allowed to settle. If the solution is translucent and there is still drying agent flowing
around in the mixture, the solution is reasonably dry. If this is not
the case, the mixture is allowed to sit a couple of minutes and then
re-examined. If the mixture is still not translucent, a little bit more
drying agent is added and the procedure above repeated.


Wet solution
Dry solution
Why does my solution start to "boil"?
Depending on how much water remained in the solution, the solvent can boil if too much drying agent was added at once. This is particularly a problem for low boiling solvents like diethyl ether and dichloromethane, where one can often observe the formation of bubbles in the solution due to this problem. All of these drying agents should preferably be used after the organic solution is treated with a saturated sodium chloride solution, which already removed the a lot of the water from the organic layer. It is also imperative that the organic layer and the aqueous layer are separated well!
How do I know that the solution is dry?
Small water droplets cause the mixture to be kind of milky in the beginning. Most of the time, the solution becomes translucent if it is dry. In addition, the drying agent does not clump up anymore and floats in the solution as well when the mixture is swirled.
What do I do if the solution is dry?
Since the drying process is reversible at higher temperatures, the solution and the drying agent have to be separated. This can be done using a Pasteur pipette if small solvent quantities are used (<5 mL). For larger quantities, a careful decanting works best and it is also relatively fast. In some cases, a simple gravity filtration is best to remove the drying agent. Afterwards the solvent can be removed or the liquid be distilled.
Important
Pointers
1 One of the main problems in the drying process is that the above equilibrium is shifted to the left if the mixture is heated (increase in entropy!). Therefore, the drying agent has to be removed (by filtration or decanting) from the dry solution prior to removing the solvent.
2. The drying agent is only working if it is still anhydrous. This implies that YOU close the container that is used to store it right after you removed what you need for yourself.
3. Students usually tend to add way too much drying agent. The proper way of using a drying agent is as follows:
a. Separate the organic layer cleanly from the aqueous layer. You should not observe any visible water droplets or a second layer in your beaker/Erlenmeyer flask that holds the solution to be dried. If there are droplets present, you will need to transfer the solution again.
b. Add a small quantity of drying agent (~2-4 microspatulas for micro-scale setup) and swirl the mixture. The mixture should become translucent and the drying agent is still free flowing.
c. If not, wait for ~2-3 minutes and check again. If the solution is not translucent and/or all your drying agent is clumped up, add an additional (small) quantity of drying agent.
d. When the solution is dry, decant (=pour) the liquid part into another dry container and leaving the solid behind in the first flask. Alternately use a gravity filtration using a Pasteur pipette with a cotton ball (not vacuum filtration!) to remove the drying agent.
e. Remove the solvent by evaporation in a water bath or on a hot plate (depending what the procedure asks for).
4. Try to avoid a large excess of drying agent since it will
inevitably lead to the loss of product. There is a competition of water or your compound absorbing on the drying agent. Generally, water has a higher affinity towards the drying agent, but a large excess of drying agent also causes
the target compound to absorb. Thus, it might be good protocol to
extract the drying agent with some clean solvent. This solvent should be combined with the first part.
5. The proper way of describe the step in your report is "the solution was dried over [drying agent X]".
What is wrong with this picture?
